May 29, 2018 PAP-Q2-18-NI-002
NICE INSIGHT OVERVIEW: Market Growth
The market for biologic drugs is predicted to continue its strong growth over the next several years. According to Mordor Intelligence, the global market will expand at a CAGR of 8.5% from 2018-2023, reaching a value of $341.16 billion by 2023.1 Because biopharmaceuticals can theoretically modulate any physiological pathway that is well understood and treat previously untreatable diseases, significant further development of biologics is anticipated. Patent expirations are also creating opportunities for biosimilars.
Reducing costs and increasing the speed at which new drugs are developed and commercialized will remain a top issue for the biopharmaceutical industry going forward. Manufacturers are achieving these goals through the implementation of new technologies that allow greater efficiency and productivity.2 For instance, the construction of multi-product and flexible manufacturing facilities and the adoption of single-use technologies are both increasing. Biologics producers are also constantly seeking new solutions to increase the titer and yield of their processes and implementing autorotation, monitoring and process— control technologies to improve the efficiency of their operations. Interest in continuous bioprocessing for both upstream and downstream operations continues to grow as well.
Robotic and cognitive automation (RCA) is also being used to streamline resources across the clinical trial value chain, according to Deloitte.3 According to the firm, RCA has the potential to accelerate clinical trial site selection, support site initiation and improve site monitoring. Separately, natural language generation (NLG) will be employed to automate safety and efficacy sections of dossier submissions.
One of the greatest attractions of biologic drugs is their ability to offer targeted treatments. This ability ties in well with the growing interest in the development of treatments for rare diseases. With low development costs and high market value, rare disease therapeutics are attractive. To date, however, treatments for less than 1% of the world’s population have been developed.4 The pipeline of rare disease drugs is full, with many new biologic candidates developed as the result of breakthroughs in gene therapy, nucleic acid therapy and gene editing.
Numerous biotech startups are working on nucleic acid therapeutics, including DNA delivery, DNA modification (gene editing, gene therapy) and modified RNA/mRNA technology companies. Gene therapy is exciting because it has the potential to cure diseases that are considered chronic conditions that would require ongoing, long-term pharmaceutical care in a single treatment. Gene editing — notably CRISPR–Cas9 technology — is playing a key role in the advancement of many different gene and cell therapies. Rapid gene sequencing and next-generation sequencing machines are also enabling the development of new drugs, the use of genetic markers and genetic background information to select treatments and true precision medicine.4
Cell therapies will also have a tremendous impact on the biopharmaceutical industry in the years to come, as many new treatments progress through late-stage clinical trials and receive market approvals. Both major biopharma firms and emerging biotech companies are investing in CAR T-cell targeted therapies, particularly for the treatment of oncology indications.
Rapid advances in immunotherapy treatment are also taking place, many with the goal of preventing diseases.5 While most of the drugs under development today are focused on the treatment of cancers, companies are also investigating their application for the targeted treatment and prevention of other chronic conditions like diabetes, cardiovascular diseases, Parkinson’s and multiple sclerosis.
Electrical management of the body using bioelectronics and electroceuticals has the potential to impact the way many diseases will be treated in the future.4 Galvani Bioelectronics, the joint venture between GSK and Google, is one company developing miniature devices designed to change nerve electrical signals. Investments in the brain machine interface (BMI) and smart implantables (enabled by extreme miniaturization of electronics) for targeted drug delivery when needed (via monitoring using sensor technology) are also increasing. Digitally-enabled pharmaceutical drug/device combinations that serve as closed-loop systems for automatic dosing could potentially change hospital and subacute care. Such devices could also allow consumers to detect disease states much earlier and thus receive more effective treatment before a disease progresses.

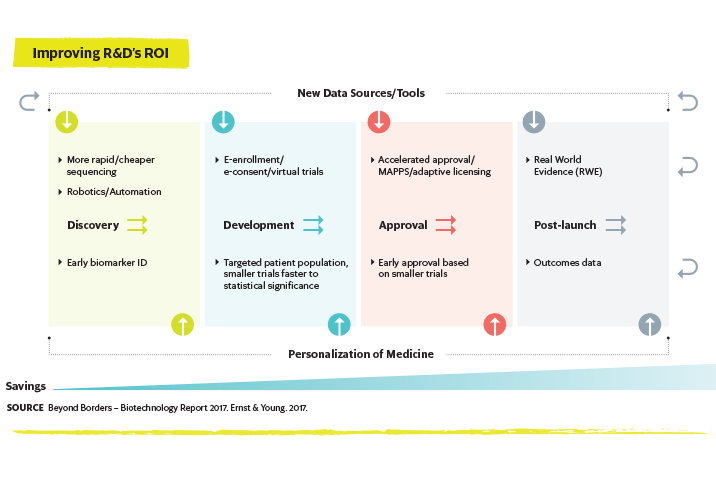
Access to real-world evidence (RWE) may dramatically impact new drug development and facilitate clinical trial setup.3 Using data in new ways may also have the potential to move treatment to the pre-disease state.6 Data obtained from genetic
mapping can be linked to observations of disease characteristics, leading to the identification of new diagnostic markers. When combined with clinical lab results, real-time data generated from wearables and other mobile technologies, behavioral data from social media sites and data on how patients on existing drugs are responding, genetic information can expand the applicability of precision medicine and, perhaps, even enable predictive medicine.
Artificial intelligence (AI) can be used to analyze large sets of pharmaceutical data — whether research results, manufacturing process information, preclinical study data, clinical trial results or patient treatment responses — and thus has potential to impact all aspects of drug discovery, development and commercialization. Perhaps most significantly it may help reduce the cost of R&D activities by driving greater efficiency.6 For instance, AI can help identify patterns and links across large sets of data to rapidly identify potential drug targets. When combined with high-throughput technologies and cloud-based data-sharing platforms, R&D is accelerated through both greater productivity and increased collaboration.
Traditional biopharmaceutical companies are facing real competition from players outside the industry with expertise in digital technologies.6 Technology companies like Apple and Alphabet (the parent of Google), along with wellness firms and other nontraditional organizations with access to consumer data, an understanding of consumer behaviors and advanced digital and big data technologies, are positioned to compete or partner with biopharma companies in the development of new digital medicines.6 Collaborations, in particular, have the potential to address real issues in the biopharma industry, such as chronic disease management/treatment, patient adherence and clinical trial design and implementation.
Some companies are already taking action, including Sanofi with Verily Life Sciences, the life sciences unit of Alphabet, and Novo Nordisk with IBM Watson Health.6 The former two companies agreed in 2016 to invest $500 million in the development of diabetes solutions that combined devices, software and medicines.5 Another example is device maker Medtronic’s collaboration with technology company Qualcomm for the development of a continuous glucose monitoring system that provides patients and providers with information that they can act upon.
 Faster Pace of Innovation
Faster Pace of InnovationRegardless of whether new innovations are coming from traditional big biopharmaceutical firms, startups established in a garage or nontraditional technology companies, they are coming at a faster pace than ever before.3 Patients with access to greater data and the use of AI and augmented virtual reality are leading to new innovations, many of which facilitate the development of personalized medicines.7 Disruptive digital technologies are being adopted and implemented that will dramatically change the way drug discovery, clinical trials and patient management are performed. Rapid advances in genetics, gene editing, cell and gene therapies, electroceuticals and smart implantables are already changing the way diseases are treated.3 Predictive medicine and the use of increasingly targeted therapies will enable safer and more effective early treatment and, ultimately, disease prevention.5

Mr. Walker is the founder and managing director of That’s Nice LLC, a research-driven marketing agency with 20 years dedicated to life sciences. Nigel harnesses the strategic capabilities of Nice Insight, the research arm of That’s Nice, to help companies communicate science-based visions to grow their businesses. Mr. Walker earned a bachelor’s degree in graphic design with honors from London College of Communication, University of the Arts London, England.