June 10, 2021 PAO-06-21-CL-04
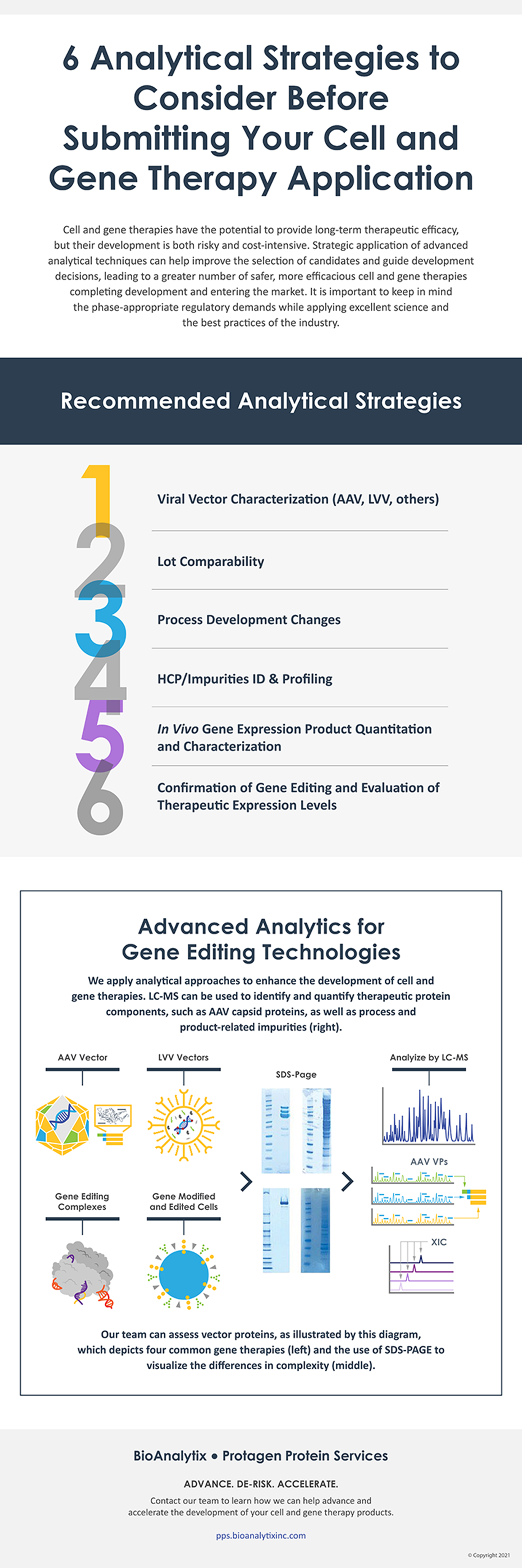
High-resolution LC-MS based characterization, quantification, and in vivo expression profiling approaches are increasingly being applied to enable improved program understanding, controls, and clinical outcomes assessment. Each analytic service program at BioAnalytix is customized to suit their client’s particular molecules and the phase-appropriate regulatory demands while keeping with excellent science and the best practices of the industry. In this infographic, learn the six advanced analytical strategies to consider before submitting your cell and gene therapy application. Be sure to reach out to their team for a strategy call if you have any questions: contact@bioanalytixinc.com.

Nice Insight, established in 2010, is the research division of That’s Nice, A Science Agency, providing data and analysis from proprietary annual surveys, custom primary qualitative and quantitative research as well as extensive secondary research. Current annual surveys include The Nice Insight Contract Development & Manufacturing (CDMO/CMO), Survey The Nice Insight Contract Research - Preclinical and Clinical (CRO) Survey, The Nice Insight Pharmaceutical Equipment Survey, and The Nice Insight Pharmaceutical Excipients Survey.

BioAnalytix works with leading biotherapeutic companies in the development and application of advanced analytics and data analysis of complex biologics, from candidate selection through regulatory package development. Applying state of the art mass spectrometry, biophysical analytics, and method development expertise, our team of PhDs provides precise data packages for specific project needs through all stages of biotherapeutic drug development. We further offer our insights and understanding of CMC and regulatory requirements to support product development from IND through BLA.