May 12, 2021 PAO-05-21-CL-03
Avid Bioservices began in 2002 as a subsidiary of an R&D-based pharma company focused on bringing its own drugs to market. Avid initially offered manufacturing services to customers to leverage excess capacity. Ultimately, the board of directors determined that the best way forward was to transition the company into a dedicated CDMO focused on biologics, while bringing our unique experience as an innovator to support client programs.
The transition took effect in January 2018 and involved changes in both personnel and culture. While some of the attributes of a biopharmaceutical company are useful for a CDMO, for the latter there is stronger emphasis on manufacturing, project management, and streamlining of internal processes. This required a cultural shift more toward execution (on-time, in-full, and in-specification delivery), as well as a customer-centric approach to what is essentially a service business — albeit one requiring a high degree of technical competence. To that end, over the last three years, Avid has worked to bring in the capabilities, systems, and processes required of high-performing CDMOs and combine them with the existing, valuable aspects of the business, not least of which is a 16-year track record of commercial manufacturing.
Even though Avid has been operating as an independent business for just three years, we are a top-tier, world-class commercial business. Throughout our full history, we have manufactured nearly 200 commercial mammalian cell culture batches, with zero 483s resulting from our last five FDA inspections, the most recent earlier this year. To the best of our knowledge, there are fewer than a handful of CDMOs globally that have made so many commercial batches throughout their history, and even fewer with an equivalent regulatory track record.
We are thus in a unique position as a relatively new name to the market, but with experience matched only by the largest, most well-established competitors — and this is combined with an extraordinarily high degree of touch. We are also beginning to realize the benefits of our customer-centric approach and processes, IT infrastructure, and other investments that have been implemented to support that strategy. It may not be the flashiest or most attention-drawing attribute, but repeatedly executing on time while meeting all specifications is the core criterion of an effective CDMO.
One of the biggest changes that enabled Avid to successfully transition to a dedicated CDMO was the restructuring of the company’s board of directors and the management team. Today, the board comprises top leaders in the CDMO sector. Every member of this extraordinary group brings a wealth of industry experience and their own unique perspective on the business, while remaining refreshingly absent of ego.
That unparalleled experience is central to what differentiates Avid from our peers and drives our success as a CDMO. One of the first courses of action the board took was to bring in new management with the ideal experience, skills, and abilities to determine where the business should go next and how to take it to that next level. Daniel Hart was brought in as the chief financial officer and Timothy Compton as chief commercial officer, and Richard Richeri returned to the company as chief operations officer, as well as myself as chief executive officer. The team is filled out with legacy Avid employees, such as Ray Marzouk as vice president of quality. We all have worked in the contract manufacturing space for many years, and each of us has the particular skills and experience needed to be effective at our individual jobs. Most critically, we all work collaboratively and successfully as a team.
With the management team in place, it was possible to map out a plan forward and rapidly begin implementing the changes needed to ensure success. The team concept at Avid, however, goes beyond the leadership. Our success has been underpinned by all of Avid’s employees, who are also highly skilled and experienced and committed to our mission of improving patient lives by consistently delivering high-quality biopharmaceutical products. It also helps that everyone understands that we stand and fall on our combined performance and not that of a single individual or even a department. The COVID-19 pandemic and its ongoing impacts have highlighted even more the importance of teamwork, and we have seen individuals and groups stand in for others — even in different departments — to help when colleagues have needed to quarantine or in some cases deal with COVID-19 personally or in their family. As we all look forward, there appears to be a light at the end of the tunnel, certainly in our case. Employees are feeling much more settled as more than 75% of the workforce has been vaccinated, and that number continues to climb.
That unparalleled experience is central to what differentiates Avid from our peers and drives our success as a CDMO. One of the first courses of action the board took was to bring in new management with the ideal experience, skills, and abilities to determine where the business should go next and how to take it to that next level.
 Growing Value of Proximity
Growing Value of ProximityProximity to existing and potential clients has always been an advantage in the CDMO industry, and it has become ever more so in the face of the COVID-19 pandemic. During tech transfer, customers want to have people on site and to be in constant communication with their provider’s teams. The same is true if unexpected challenges arise. A large time difference between customer and CDMO will mean communication requires one side to work at night; while typically not much of a hardship for sporadic calls, time differences become more impactful in situations requiring frequent communication.
The situation has become far more complicated now due to the travel limitations created by the pandemic. People don’t want to fly from the United States to Europe or Asia — if such travel is currently even permissible at all — if they then would have to quarantine for two weeks before visiting a CDMO site, and then two weeks more once they return home. Additionally, changing restrictions may mean that a long-planned and critical visit may become impossible at the last minute, wreaking havoc on timelines. Streamlining or abbreviating the supply chain is a natural result that is shifting global strategies.
Our Tustin, California facility and operations are ideally located within one of the most active and innovative corridors of the industry. As such, Avid is well positioned to serve customers all across the country, particularly since these groups do not need to rely on virtual tours in place of site visits or face challenging time differences with respect to communication.
The strategic and operational changes at Avid — combined with the good market dynamics of the biopharmaceutical sector —have resulted in significant growth for the company. In addition, demand for biologics is expected to expand at a healthy pace over the coming decade. Part of that is due to the production capacity required for COVID-19 vaccines and therapeutics, but the aging of the population and the increasing incidence of chronic illnesses in developing markets, among many other factors, continue to drive demand for all types of biologic drugs. Furthermore, COVID-19 may be with us for some time to come, as it remains unclear how long we will be living with the impact of the pandemic and the viral variants we are already seeing appear, and in all likelihood another virus — whether a coronavirus or other class of virus — will emerge.
Fundamentally, the number of biotherapeutics being developed, the number of successes being reported out of clinical trials, and the number of approvals being granted — when considered in light of the existing and announced new capacity in total and the small number of players in the CDMO market — bodes quite well for the future of Avid Bioservices.
Indeed, through the first three quarters of our fiscal year, we have experienced 45% year-on-year growth and gone from a loss-making company to a profitable one — a transition that occurred in one quick jump rather than via a long crawl. We are signing ever-larger volumes of new business, such that our backlog has also increased by more than 100% over the same period. This level of performance is phenomenal and reflects not only the state of the market as well as our successful evolution into a dedicated CDMO, but also the excellent work of Avid’s business development team.

 Expanding Capacity to Meet Growing Customer Demand
Expanding Capacity to Meet Growing Customer DemandOur strategy at Avid is to stay ahead of the demand curve as much as possible, while maintaining an appropriate balance of used and available capacity. In so doing, we not only offer capacity to onboard new clients, but also provide peace of mind to our existing clients that we can support their success as they navigate the various phases of clinical development towards commercial approval.
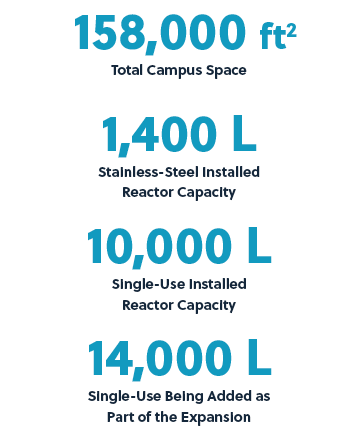
With our backlog increasing, we determined that it was necessary to increase our existing capacity. We recently raised capital with the goal of implementing a two-phase expansion at our Myford facility. The first phase was initiated in November 2020 and is expected to come online in early 2022. It involves debottlenecking the downstream processing operations with the addition of a second purification suite and will increase our Myford North facility capacity, which translates to approximately $50 million in increased annual revenue potential.
The second phase (Myford South) began in February of this year. It involves the addition of a second manufacturing train with both upstream and downstream processing suites within the Myford facility. Myford South will leverage many common utilities inside the same Myford shell. Once completed, it will add approximately $100 million of additional annual revenue potential and 14,000 L of single-use bioreactor capacity.
Both phases of the expansion leverage our existing blueprint, but with some improvements based on lessons learned. The Myford North facility was originally constructed in a modular manner using only single-use equipment, and all additional equipment in our expansion phases will be single-use, implemented in a modular design. With these new areas, we are exploring the incorporation of automation and other improvements that can provide process and economic efficiencies for commercial clients that require multiple batches of the same product. We have also been strengthening our internal systems by installing next-generation, integrated enterprise resource planning (ERP), quality management systems (QMS), and laboratory information management systems (LIMS). Going forward, we will continually seek process improvement, both in the biologics processes we develop, in the technologies we are able to employ, and in our own internal processes that support and enable us to deliver high levels of customer satisfaction.
As our current business, which is focused on recombinant proteins and antibodies, is on a solid foundation and continues to generate significant growth, Avid intends to look to other areas where we feel we can leverage our skills and expertise and the value proposition we have honed for customers. One of those areas may be backward integration into cell line development. We would also like to further expand our process development capabilities to better serve our clients, because process optimization is always a pain point in the biopharma industry and is often sacrificed as clients are forced to balance costs, risks, and timelines. There is an opportunity for good CDMOs to improve on the current process development approach, and I would like to see Avid take the lead in this area.
Our existing market offers significant opportunity to grow the business, but we believe Avid’s value proposition and technical competence can also translate to other adjacencies within the biologics field, where we see customers who would value high-quality cGMP production capacity and capabilities, a solid regulatory approach, technical competence, customer-centric culture, and a true partnership approach to business. Going forward, we intend to evaluate these and, based on those evaluations, we will determine future steps for Avid’s development.

Nick currently serves as President & CEO of Avid Bioservices and previously served in senior executive roles for several contract manufacturing organizations and life sciences companies, including President & CEO of Therapure Biopharma and Evolve Biologics, Managing Director of Nipa Laboratories Ltd, Head of the Life Sciences Division of Clariant International Ltd, President and CEO of Rhodia Pharma Solutions and President of Codexis Pharma Division. Up until recently Nick was also a Board Member at Induce Biologics Inc and Redrock Regeneration. Nick holds a BSc (Hons) in chemistry from Queen Mary University of London and an MBA from the University of Huddersfield in the UK.