September 15, 2017 PAP-Q3-17-FA-002
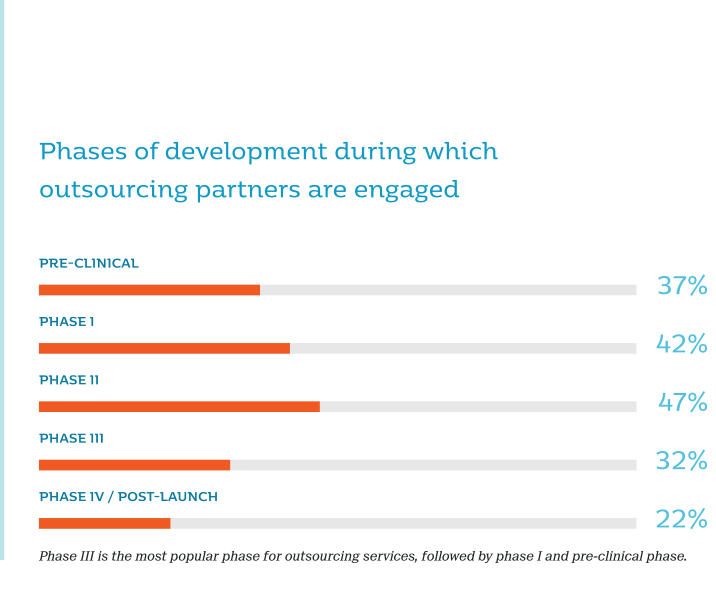
Driven by the growing number of clinical trials and demand for advanced medicines across the globe, the CRO market is projected to reach $59.42 billion by 2020 at a compound annual growth rate (CAGR) of 9.8% from 2015 to 2020, according to Zion Market Research.1 Valued at $34 billion in 2014, clinical trial services accounted for over 70% of the market value; services for phase II to IV trials generated 60% revenue. By 2020, 72% of clinical trials are projected to be outsourced.2 In addition, the 2017 Nice Insight Preclinical and Clinical Contract Research Survey demonstrated that the most popular development phase for outsourcing services was phase II (47%), followed by phase I (42%), pre-clinical (37%) and phase IV/post-launch (22%) in 2017.3 Geographically, North America and Europe represent the two largest regional CRO markets, holding a 43% and 40% global share, respectively.4 In addition, the clientele for CROs have expanded from traditional pharma, biotech and medical device industries to government institutions, foundations and universities.
The global CRO market is led by a number of large CROs (e.g., Covance, Quintiles, Parexel), which capture over 50% of market share.5 CROs are under the constant pressure to expand their service portfolio and geographical presence. To meet this challenge, mergers & acquisitions, strategic alliances and joint ventures have become the norm in the CRO industry. In recent years, going public has become another strategy to achieve growth.1 As a result, large CROs become larger with full-service capabilities and extensive global reach. Smaller CROs try to differentiate by focusing on niche area specialization or functional services such as bioanalytics, eClinical solutions and HEOR (health economics and outcomes research).6
Currently, emerging countries account for 15.7% of the global CRO market.2 Most of the worldwide population lives in emerging market countries and several of these countries have a rapid growing economy. China and India are the exemplars for a large population and growing economy — they present tremendous opportunities of high market growth and financial returns for both pharmaceutical and CRO industries. Rising personal income levels, increasing incidence rate of chronic and lifestyle diseases (i.e., cancer, diabetes) and demand for new drug products have been driving the vigorous growth of these markets in the 21st century.
Outsourcing services in emerging countries are expected to grow at a higher rate than in developed markets. By 2020, the emerging countries will harvest over a quarter of global CRO market share.2 To enter the emerging markets, some pharmaceutical companies have opened their own R&D and manufacturing facilities, while others have sought the assistance of global and local CROs.
The pharma industry has been cautious in terms of engaging emerging market CROs. According to the Nice Insight 2017 Preclinical and Clinical Contract Research Survey, the willingness to consider emerging market CROs varies among different pharma/biotech sectors with big pharma/biotech showing the highest consideration rate of 62%, followed by small (54%), mid-sized (51%), and emerging (44%) pharma/biotech sectors. A total of 59% of the respondents who consider emerging market service providers have indeed worked with CROs in these regions. Quality is the greatest concern that hinders engagement with local CROs. Concerns with regulatory compliance, logistics, intellectual property and communication constitutes other major barriers.3
For clinical trials, emerging markets offer several appealing features, such as significant savings in clinical trial costs of 40%–60% compared to the cost of clinical trials conducted in developed countries.5 Other features include the ease in patient enrollment, a skilled scientific research and healthcare working force, and government incentive programs (i.e., tax exemptions).1 Consequently, more large-scale multi-site clinical trials have been conducted in these regions.
One approach to improve clinical study efficiency has been to conduct trials in developing countries. As a result, clinical trials have been shifting from western countries to emerging markets. To date, the United States and Europe still host the largest proportion of clinical trials. However, Asian and Latin America/the Caribbean has witnessed the fastest growth in terms of number of clinical trials: an average annual growth rate of 30% and 12%, respectively, between 2005 and 2012 in contrast to an average annual growth rate of 2% in the US in the same period.7 Operating clinical trials at a global scale can be a daunting task. The pharmaceutical industry has relied heavily on outsourcing to reduce costs and shorten development timelines. To this end, selecting a CRO has become extremely critical to the success of an entire study. In recent years, the industry has witnessed a number of strategic partnerships formed between global pharmaceutical companies and global CROs to streamline clinical development processes. A prime example of this practice is Bristol-Myers Squibb and Pfizer’s partnerships with Icon and Parexel as their strategic providers of clinical-trial implementation services in June 2010 and May 2011, respectively.8 A recent alliance between Takeda and PRA Health in 2016 has taken the sponsor-CRO strategic partnership to an unprecedented level of integration of the two businesses, which may serve as a platform for future pharma-CRO partnerships.9 To meet sponsors’ needs to conduct clinical trials efficiently within a tight timeline and deliver high-quality data, CROs are forming preferred provider relationships with investigation sites, investigator networks and site management organizations (SMOs).10,11 The healthy working relationship between CROs and the sites can eventually translate into better enrollment forecasting, faster study startup, improved protocol and data quality. Parexel’s Global Site Alliance Network and INC’s Catalyst program are two examples of this proactive approach for nurturing CRO-investigator relationships.10,11
At present, sponsor-CRO relationships comprise a combination of tactical, preferred provider and strategic partner relationships. Again, according to the 2017 Nice Insight Preclinical and Clinical Contract Research Survey, being a preferred provider is the most common type of outsourcing relationship applied in 50% of outsourced projects; 25% are contracted to tactical service providers and strategic partners, respectively. For CROs, becoming a strategic partner is a gradual evolvement from tactical service provider status. Overall, 62% of respondents agreed that a CRO that started off as a tactical service provider would become a preferred provider, and 68% felt it was highly likely that a preferred provider would become a strategic partner. Additionally, respondents showed strong interest (84%) in establishing a strategic partnership with a CRO in the next 12-18 months.3
A successful strategic partnership is mutually beneficial, requiring commitment from both parties to work together as a united team with integrated goals, structure and processes.12 This partnership must be built upon a shared vision, as well as mutual trust and respect. It is important to clarify expectations, responsibilities, commitments and investments for each party at the very beginning of the partnership. Additionally, a governance structure is fundamental for communication, collaboration, escalation and risk management that enables the sponsor and CRO to function as an integrated team. Another key element for a successful strategic partnership lies in effective, open communication that promotes a high degree of cross-organizational transparency and information sharing.

Dr. Challener is an established industry editor and technical writing expert in the areas of chemistry and pharmaceuticals. She writes for various corporations and associations, as well as marketing agencies and research organizations, including That’s Nice and Nice Insight.