October 12, 2021 PAO-10-21-CL-04
In recent years, the focus of respiratory development has realigned to nurture lesser known indications with unmet needs. Examples of these rare diseases include progressive fibrotic interstitial lung diseases (PF-ILD), pulmonary arterial hypertension (PAH), cystic fibrosis (CF), and non-cystic fibrosis bronchiectasis (NCFB). Within more prevalent conditions, such as asthma and COPD, sponsors are increasingly focused on smaller subpopulations of patients who have a specific clinical or biomarker phenotype or whose disease burden remains high despite available therapy. Using biomarkers can aid the drug development process by reducing the time needed to bring a product to market.
Respiratory studies have an additional layer of complexity in that they do not typically involve healthy volunteers. In these cases, study populations may include patients with various levels of disease burden — even those with severe situations, such as end-stage respiratory failure. Therefore, in a traditional respiratory clinical trial model, accessibility is typically low while patient burden is very high.
The combination of studying smaller subpopulations, varying levels of disease complications, and increased patient burden has resulted in the need for a more flexible protocols utilizing digital and decentralized options to improve the accessibility of clinical trials for all, ultimately bringing the study directly to the patient.
In the years leading up to the COVID-19 pandemic, PPD made strategic investments together with the creation of a specialized unit known as PPD® Digital. This Center of Excellence (CoE) specializes in digital and decentralized trial design and technology. The team comprises a variety of experts who contribute to the operations, management, strategy, and design of DCTs.
Throughout this journey, we have helped sponsors transition from traditional clinical trial models into custom-fit decentralized solutions, using tools such as e-Consent, eCOA, telemedicine, and home healthcare visits with respiratory nurses to achieve acceptable endpoints with real-time data.
While there was increasing focus on how decentralization could be applied before the COVID-19 pandemic, many sponsors remained cautious or reluctant to apply these innovations. Historically, this is because DCTs have been perceived as difficult to operationalize and involved further consideration from leading regulatory bodies. However, the pandemic shifted the conversation from decentralization as an option to consider to an essential solution, creating a pivotal moment for the industry as a whole. PPD Digital has seen firsthand the transformation from using these solutions as an application to “rescue” ongoing trials into a tailored and proactive application of innovations for planned trials. This trend will continue to accelerate as the range of providers and solutions expands, and as the industry now has the experience and confidence to see these innovations as tried and tested solutions.

Adaptability must be built into clinical studies to provide the needed flexibility for patients who exhibit different needs, particularly those whose respiratory diseases are unpredictable and whose treatment requirements are just as complex. Technology must be woven into the layers of the human element involved.
As an example, clinical trials in asthma increasingly focus on rarer subpopulations with more complex drug administration and thus face unique challenges, such as higher screen failure rates and fewer patients living close to the site. In addition, these patients are typically working age and often have career responsibilities, so frequent on-site assessments for investigational product (IP) administration or spirometry may not be compatible with their lifestyle. Decentralized strategies can help overcome many of these issues.
Special considerations for the execution of decentralized respiratory clinical trials include standardizing rater training, diagnostic criteria, mobile spirometry, and outcome measurements.
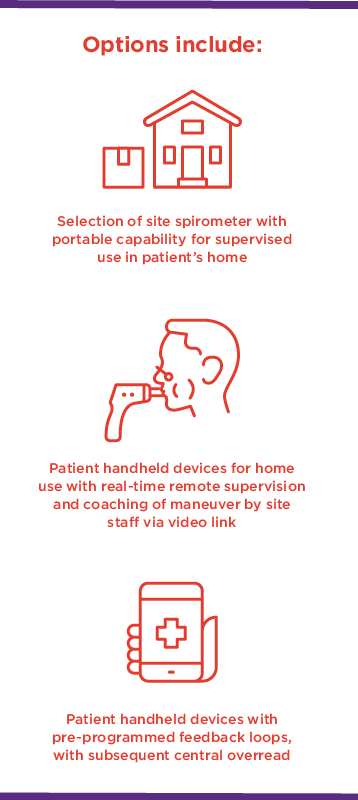
Spirometry has traditionally been done at sites with centralized equipment and direct observation by site staff. This model ensures confidence in data quality but is less patient-centric, in that it requires patients to make frequent visits to a site. For many visits, spirometry is the sole driver behind the need for an onsite evaluation. While handheld 3G-enabled tools to collect peak expiratory flow (PEF) have long been applied in asthma trials, allowing real-time daily PEF capture, tools capable of collecting forced expiration (FEV1) and forced vital capacity (FVC) data at home have been limited until now. This transition from performing spirometry in the clinic to in the home is one of the last milestones in the decentralization of the respiratory clinical trial space.
In addition to these remote solutions allowing a reduction in onsite visits, they also allow sponsors to collect more frequent spirometry assessments, with the potential reduce variability in the data.
Another modern tool showing increased use in respiratory research is wearable technology. For example, a sensor unit that looks and feels like a wristwatch can enable actigraphy, a non-invasive method of monitoring human rest and activity cycles. This allows for gross motor activity to be measured in real time, along with other data, such as light exposure. These data are transmitted to clinicians and study teams to objectively measure patient functional status in their environment in a manner that was not previously possible. These are arguably more relevant and timely endpoints than others collected via traditional modes. In respiratory trials, these sensors would aid in the detection and classification of minimal clinically important differences (MCIDs) as patients are increasingly tasked with reporting their data remotely.
Adaptability must be built into clinical studies to provide the needed flexibility for patients who exhibit different needs, particularly those whose respiratory diseases are unpredictable and whose treatment requirements are just as complex.
The impact of adherence on patient outcomes has long been recognized in respiratory trials, but to date most studies still have only limited data sets. This includes patients using diaries to self-report the number of inhalations they have taken or to confirm that they have taken study medication. For some, rescue medication recall may be poor, and there is no understanding or visibility of when the patient is using the therapy. This has left many sponsors wondering if they can do more to increase visibility into medication use by participants.
Understanding adherence and use would enable earlier intervention to remind patients to comply with study dosing schedules or to detect patients relying on increasing amounts of rescue medication and flag those patients who need immediate clinical assessment. Automated, 3G-enabled smart pill containers not only can remind patients to take their medication but can also record and transmit time- and date-stamped confirmation of when pills were accessed. This real-time data transfer to the site allows earlier contact with patients to understand the reasons behind any non-adherence while providing retraining as necessary.
In a similar manner, inhalers leverage sensors to enable data collection for inhaled therapies. Digitally equipped inhaler use in clinical trials is on the rise, with several solutions having been recently approved by the FDA. Most sponsors, especially those in earlier development, select Bluetooth-enabled devices that are complementary to the inhaler. However, the approval of Teva’s Digihaler®, where the Bluetooth-enabled sensor is integrated with the inhaler at the time of manufacture, demonstrates that integrated solutions are possible and forthcoming. In both of these cases, data are automatically sent to site staff via a mobile app that the clinician can review to understand the patient’s level of control, thereby forming a crucial part of a remote visit.

The 6MWT is a well-established test frequently used in trials of pulmonary arterial hypertension (PAH) and interstitial lung disease, and it can be predictive of mortality. It is used both as an eligibility criterion and as an outcome measure. Traditionally, the 6MWT has been required to be done onsite in a measured hallway and directly observed by a trained member of site staff. Challenges include the frequency of evaluations due to the requirement of rest periods between testing, variability due to effort dependence, and concerns around how a negative learning effect can impact values over time. Development and validation of methodology to enable patients to perform 6MWT remotely using smartphones, supplemented by provision of a pulse oximeter, is ongoing and may help overcome some of the challenges sites and sponsors face with test frequency, consistency, and logistics.
PPD Digital can design and integrate these types of technology into your clinical trial while following our robust onboarding and due diligence process. Our goal is to provide with excellence in all aspects of trial delivery — with the addition of PPD Digital’s decentralized expertise, we have the ability to take this to the next level.
The COVID-19 pandemic has accelerated innovation across all therapeutic areas — but most specifically around how remote technology can improve the patient experience, site interface, and patient health in clinical practice. Cystic fibrosis patient groups are at the forefront of this space and have created free apps that integrate data from multiple devices to build a holistic picture of the patient’s status, potentially allowing for more timely identification of infections and earlier intervention. An example of this is Project Breathe by the Cystic Fibrosis Trust. The Project Breathe app digitally captures variables, including height, weight, temperature, heart rate, O2 saturation, spirometry, sleep, and activity, as well as patient-reported outcomes (PROs). The addition of blood glucose monitoring and inhaler usage in the future could further expand the scope of variables captured, as well as the breadth of data sets.
Successful implementation of integrated digital solutions in the respiratory space, such as Project Breathe, should help sponsors gain the confidence to apply similar approaches within their clinical trials by providing the final reassurance that not only can it be done, but that it is also what clinicians and patients want.
Large data sets such as this could be powerful tools to demonstrate value to payers beyond conventional endpoints provided to support regulatory approvals.
While we have seen a rapid adoption of DCT solutions over the last year, it is crucial that these solutions are applied selectively and are based on detailed knowledge of how technology could strengthen endpoint collection. Our consultants work closely with sponsors to tailor solutions to the program needs and guide stakeholders away from applying solutions just because the technology exists.
PPD balances technology and innovation with risk management to meet the needs of primary stakeholders: patients, sites, and sponsors. In the past five years, PPD has conducted more than 150 studies in respiratory medicine, reaching over 42,000 patients worldwide. We are continually leveraging our vast digital and decentralized clinical trial experience and subject matter experts to establish comprehensive, flexible options for respiratory clinical trials. Connect with our team to learn how your protocol could be digitally optimized for the future.

Tim Rich serves as vice president and leads the consultancy, innovation, and strategy group, which is a driving force behind the adoption of decentralized strategies while bringing forward novel solutions. Prior to his appointment, Rich was a member of PPD’s biotech operational leadership group. In this role, he provided strategic direction, leadership, and management across multiple divisions and therapeutic areas by leveraging more than 20 years of experience in project delivery that covers a wide range of indications –- including complex rare disease and gene therapy programs. Rich joined PPD in 2006, working in project management and eventually progressing to his current leadership role. In total, his experience spans a variety of areas in the clinical trial space, such as global project management, portfolio management, development operations, and client relationship roles.