Demand for human mesenchymal stem cells (hMSCs) is increasing dramatically as numerous cell therapy, tissue engineering, and related products advance through clinical trials. Greater efficiency and productivity in GMP manufacturing will be essential to produce sufficient quantities of hMSCs at an acceptable cost for patients and payers.
Critical Raw Material
Human mesenchymal stem cells (hMSCs) are a critical raw material for many regenerative medicine products including cell therapies, engineered tissues like bones and organs, and, crucially, cell-derived products, such as extracellular vesicles and growth factors. This mammalian cell type is also instrumental in ongoing research to 3D-model diseases ex vivo or to reconstitute skeletal muscle tissue for so-called “clean” or cruelty-free meat.
A few cell therapy products have received regulatory approval around the world, and others are rapidly advancing through late-stage clinical trials. Bioprinted bones and organs and meat grown from animal muscle precursor cells derived from MSCs will require more research and technology advances to be realized, but the progress made to date suggests that multi-application success is likely to be achieved in the next decade or so.
Focusing on therapeutic applications, hMSCs have been identified for the potential treatment of many different diseases. Bone and cartilage and cardiovascular diseases and diabetes account for the largest percentage of clinical trials, but others receiving attention include Crohn’s, Alzheimer’s, hematological, lung, liver, kidney and host-graft diseases, spinal cord injury, multiple sclerosis, cancer, and many more.
A Few Growing Pains
Like other technologies, hMSC development has followed what is known as the Gartner hype cycle, in which an “innovation trigger” leads to a “peak of inflated expectations” followed by a “trough of disillusionment” and then a “slope of enlightenment” leading to a “plateau of productivity.” The hMSC hype cycle can be seen in Figure 1.1
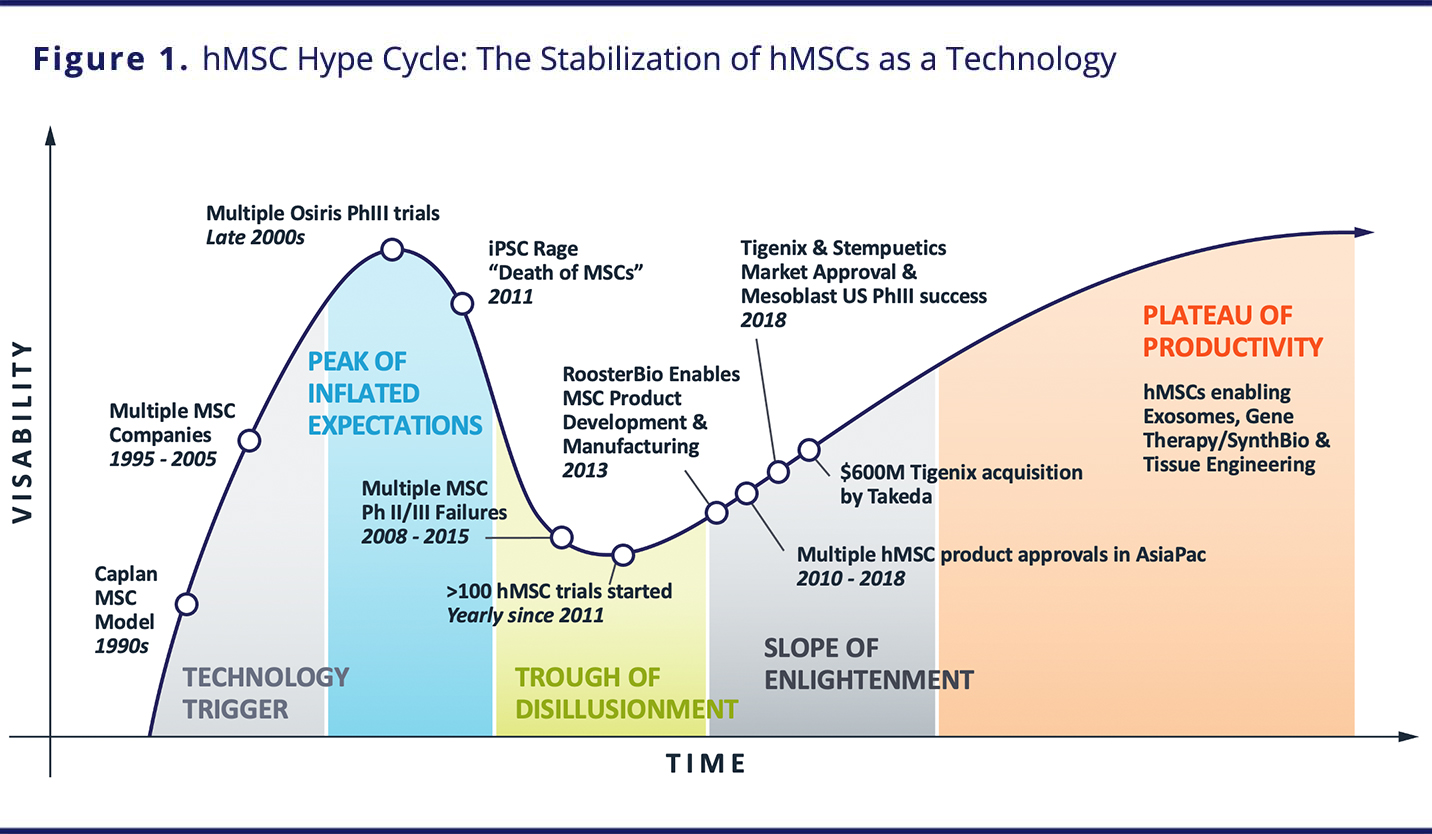
VIEW LARGER IMAGE
Results of early studies with hMSCs led to significant interest and investment in the first decade of the 2000s. Nevertheless, owing to variable manufacturing practices, differences in isolation techniques, an underappreciation of the impact of culture methods on final product quality, a lack of understanding of hMSC mechanisms of action, and poor potency assays, there have been initial failures and limited adoption. Yet in recent years, manufacturing advances, greater knowledge gained from basic and applied research, and confirmation of therapeutic properties and MSCs’ mechanisms of action led to renewed interest and the implementation of many clinical trials.
A Technology Akin to the Microchip
Given their widespread therapeutic potential for treating many indications and their usage in a variety of applications, hMSCs can be considered the “microchips” of tomorrow’s regenerative medicine products. Initially used as a biological tool to understand cellular mechanisms, hMSCs today have become fundamental components of regenerative medicine products and are needed in large quantities at pharmaceutical grade (Figure 2). On the basis of a review of the literature and clinical trial data, it has been estimated that the average academic non-clinical or preclinical study consumes just 43 million hMSCs, while a typical cell therapy clinical trial requires 60 billion hMSCs.2 Today, there are greater than 10 trillion MSCs manufactured globally per year to support these trials.
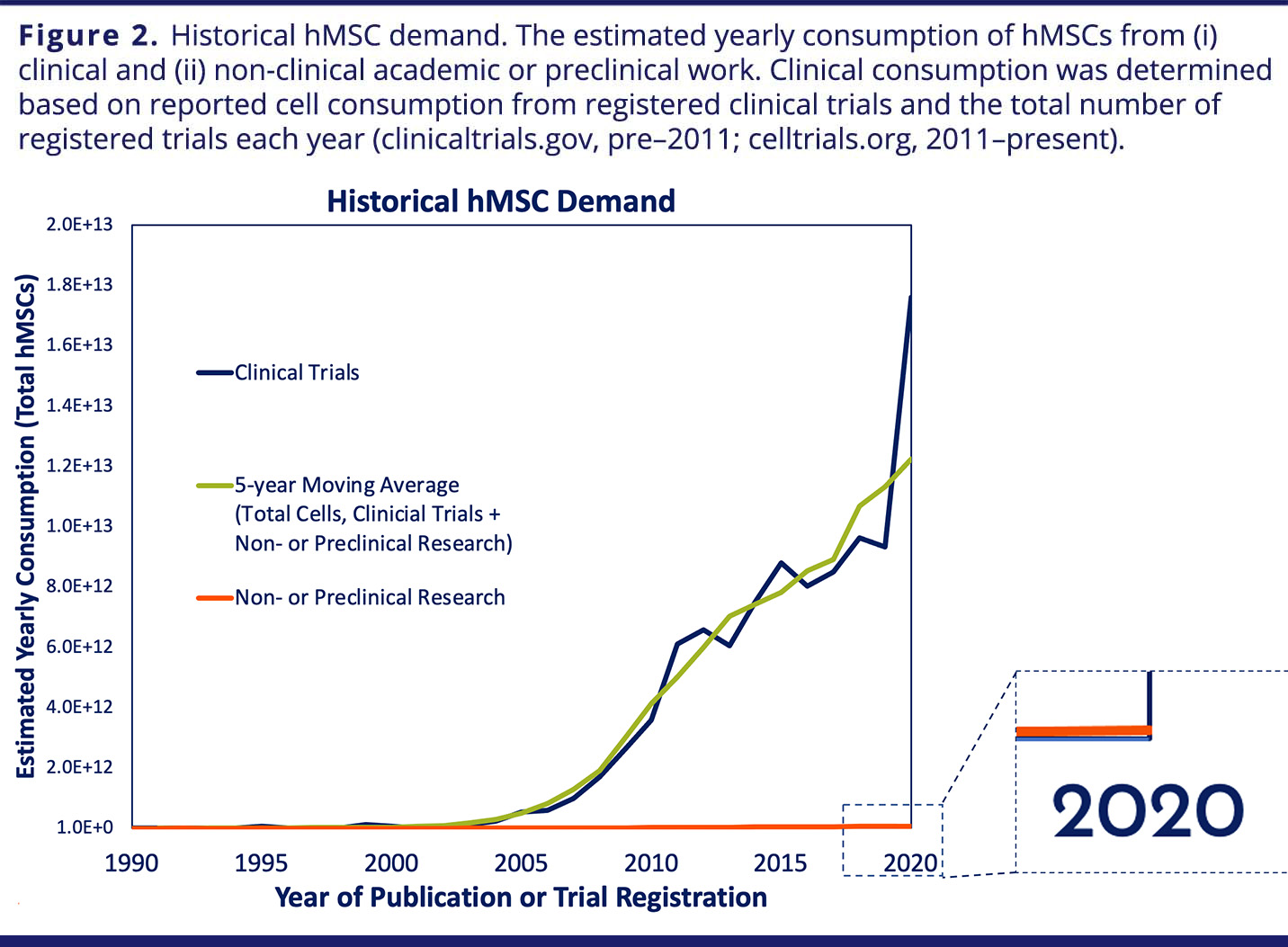
VIEW LARGER IMAGE
There is interest in accelerating the development of hMSC-based products from the U.S. Food and Drug Administration (FDA). With approvals for these therapies and engineered tissues already granted outside of the United States, a strong signal of therapeutic efficacy reported in early-stage trials, and growing momentum toward later-stage clinical trials, the potential for growth of hMSCs is tremendous. Indeed, their use in therapeutic applications is increasing exponentially in a manner similar to that observed for computing power.3
Estimating the Future Demand for hMSCs
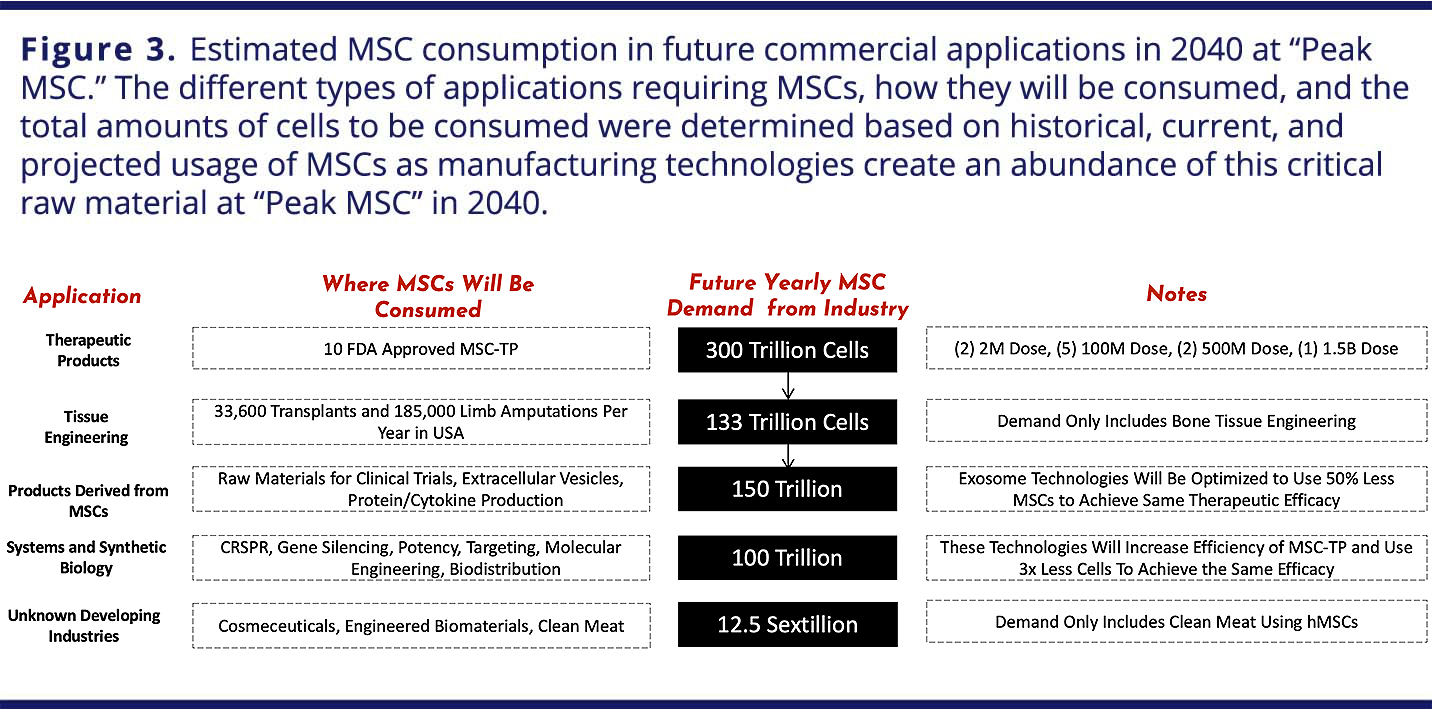
In order to estimate the future demand for hMSCs, Olsen, et al.2 considered the potential for success of different hMSC-based products and the quantity of cells required for each (Figure 3).
For cell-therapy products, given that there were 46 phase II/III and phase III clinical trials using hMSCs as of November 2017 and that the FDA estimates that 25–30% of phase III clinical drugs will receive approval,4 it can be conservatively assumed that 10 MSC-based cell therapy products will be on the U.S. market by 2030. Further assuming there will be two “low-dose” products with 2 million cells/dose, five “medium-dose” products with 100 million cells/dose, two “high-dose” products with 500 million cells/dose, and one “monster-dose” product with 1.5 billion cells/dose and 100,000 patients per each if the 10 indications, 300 trillion hMSCs would be required per year to satisfy this demand. Of course, if any of these indications involved large patient populations, such as diabetes or stroke, this estimate could increase by 10-fold or more.2
Estimates for engineered tissues and organs were based on research involving the biofabrication of half an adult femur, which requires 720 million hMSCs,5 the number of patients requiring replacement bones per year and data regarding organ transplants. Assuming that 185,000 upper and lower limb amputations are performed per year in the United States,6 with each full bone requiring approximately 1.5 billion hMSCs, 278 trillion cells would be needed per year to fulfill the demand for the all the patients requiring replacement bones.
VIEW LARGER IMAGE
While creating a functional organ via bioprinting remains a future target, it will eventually be achieved and will address a critical need for the more than 100,000 people in the United States alone waiting to receive organ transplants.7 Using the liver as an example, stem cells are known to be a precursor to the non-parenchymal cells (endothelial cells, Kupffer cells, and stellate cells) that make up 40% of the liver8 and play a functional role in liver maintenance and regeneration.9,10 In addition, only half of a human liver is required to support life and is suitable for transplant,11 and acute liver failure patients can be supported with 5–10% liver mass or a bioartificial liver composed of 10 billion cells.12,13 Assuming conservatively that 25% of the non-parenchymal cells in a tissue-engineered liver will be hMSCs, 1 billion hMSCs would be required per liver, and thus bioprinting 6,427 livers, which was the number of people at the end of 2016 waiting for a liver,6 would require 6.427 trillion hMSCs.
With liver transplants accounting for approximately 12% of all organ transplants7 — and using the same assumptions of 40% nonparenchymal cells in solid organs generated using 25% hMSCs — an additional 10-fold multiplication factor would be needed to satisfy all organ manufacturing and provide for off-the-shelf organs, or a total of 64 trillion cells.
Extracellular vesicles (EVs), such as exosomes or microvesicles collected via hMSCs, have been shown to be quite potent14–16 and under some conditions can elicit similar responses to whole hMSCs.17 Along with gene-modified or edited cells and therapies that leverage synthetic biology, EVs can be referred to as “MSC 2.0” products.2 Given their history of clinical use without significant adverse events in controlled and monitored studies, hMSCs are a relevant cell source valued for their potential in accelerating translation of EV therapies. Limited data are available on the quantity of hMSCs necessary to provide a therapeutic dose of hMSC-EVs, although an equivalent or greater amount of hMSCs for treatment appears reasonable. The same demand as for hMSC cell therapy products — ultimately, 300 trillion hMSCs per year — is therefore assumed over the next 20 years. This estimate is once again conservative, because it does not include the hMSCs required to generate other cell-derived materials, such as cytokines and cell lysates, both of which have shown therapeutic promise.18–20
Cytokines and growth factors derived from hMSCs as biological ingredients in so-called “cosmeceuticals” or bioprinted human skin models for animal-free testing of cosmetics and personal care products are both being explored by companies such as L’Oreal and Johnson and Johnson.21 Cruelty-free, “clean meat” produced from non-human MSCs, if it were to address the predicted total meat consumption, would require approximately 1.25 ×1022 cells for bioreactor inoculation.22
Peak hMSC Demand Predicted in 2040
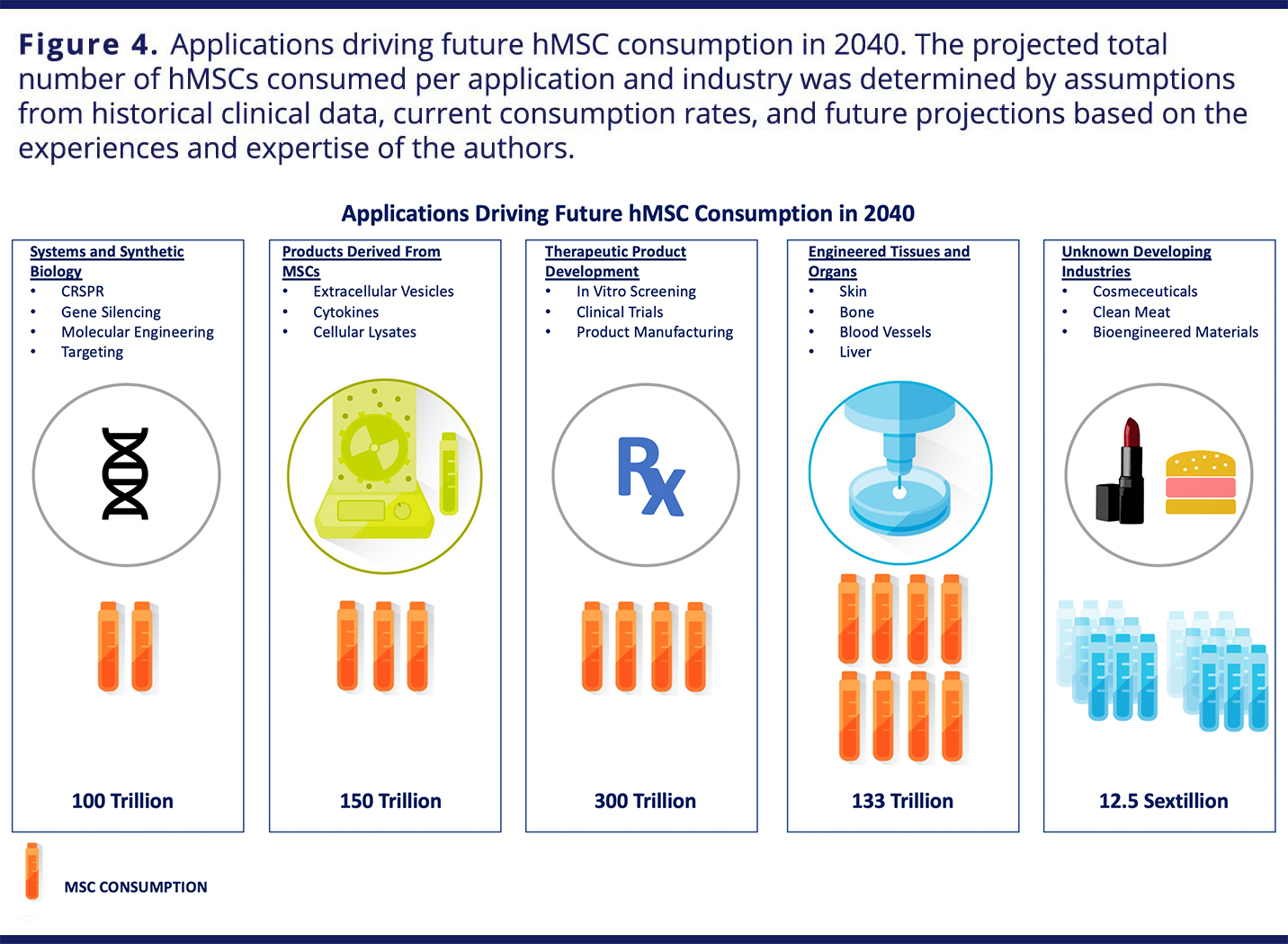
The staggering growth in demand for hMSCs is similar to that experienced for monoclonal antibodies (mAbs) over the first two decades of their development. As a result, it can be expected — as was observed with mAbs — that a variety of approved products will be on the market over the next 20 years as the industry moves toward peak MSC demand and consumption. Indeed, more than 50 products are predicted to be on the market by 2040, with approximately four products added each year — a trajectory similar to that taken by mAbs.2
VIEW LARGER IMAGE
“Cosmeceutical” products could have success initially outside of the United States, owing to the interpretation that cell products (and not the cells themselves) are the active product. Similar clinical products containing MSC-derived, “secretome”-based materials will also eventually be common (Figure 4). With FDA approvals following successful phase III trials, applications of topical MSC-derived products could find potential for discretionary, “off-label” applications in dermatologic and/or cosmetic uses by prescription. Several cell therapies based on hMSCs should be on the market by 2025, and tissue-engineered products will receive approvals by 2030. By 2040, biofabricated organs will be in clinical trials. A summary of predictions is presented in Table 1.
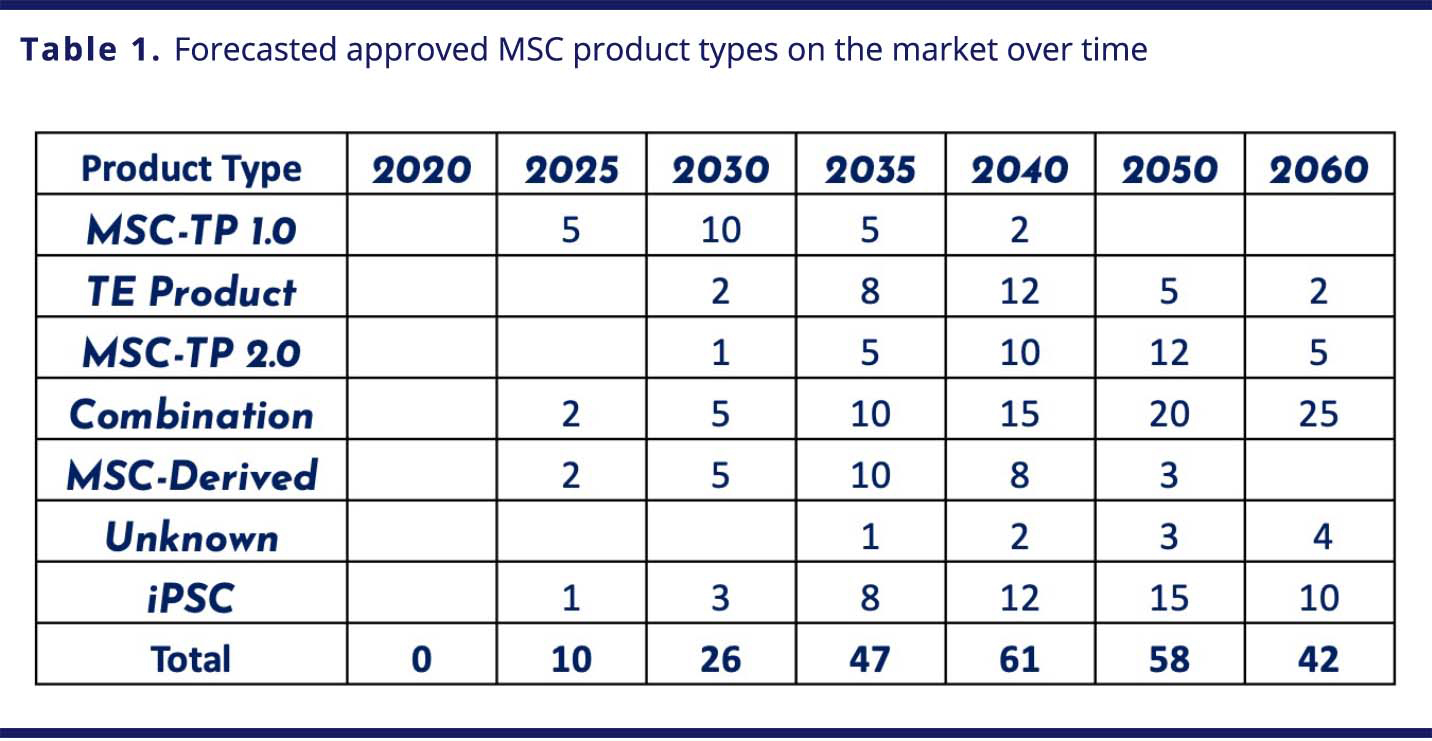
VIEW LARGER IMAGE
Success Predicated on Manufacturing Advances
Realizing this full potential will require manufacturers to develop new technologies and processes that deliver high-quality hMSCs in massive volumes—and at radically lower costs. To become a truly abundant critical therapeutic material that enables innovation at the highest level, novel manufacturing solutions will be required to support commercial manufacture of hMSC products.
Particular technical speed bumps have challenged the industry to determine a workable trypsin- and agitation-free release step from the microcarriers used to support the MSCs in order to minimize cell damage. Quenching of the proteolytic enzymes is often achieved with 10% fetal bovine serum, an animal-based product that introduces significant contamination risk. Furthermore, to isolate the suspended cells in an efficient and automated way, one must rapidly concentrate the cells while maintaining cell viability and functionality, a step necessitating filtration of the cells at large scale.2
Yet, much recent progress has been made, such as the development of new, gentler dissociation reagents and animal-free materials, now manufactured according to Good Manufacturing Practice (GMP) requirements. Completely dissolvable microcarriers are also being explored, which would eliminate the need for cell/microcarrier separation and streamline downstream processing.23
Driving Down Cost
Currently, there is a reasonable supply of high-quality hMSCs produced using robust manufacturing processes — prerequisites for clinical trials — but they come at a high cost. Scalable platform technologies are needed to economically fulfill clinically and commercially relevant lot sizes.2 An intermediate step for reducing time to the clinic will be the production of hMSCs with proven manufacturability and clinical-grade quality.
Despite the manufacturing challenges that still exist today, companies like RoosterBio® are increasing access to commercially-relevant hMSC cells through supply chain industrialization. RoosterBio has developed standardized cell bank product forms, coupled with scalable manufacturing, that includes fit-for-purpose cGMP-compatible cells and media systems supported by an FDA Master File.
VIEW LARGER IMAGE
Such solutions enable production of hMSC products in large-scale 3D bioreactors for streamlined clinical translation and reduced time to GMP product manufacturing. Overall, access to standardized, fully tested “off-the-shelf” cell banks as manufacturing starting materials for direct use in streamlined manufacturing processes provides product developers with reduced development and go-to-market timelines and, ultimately, reduced costs.24
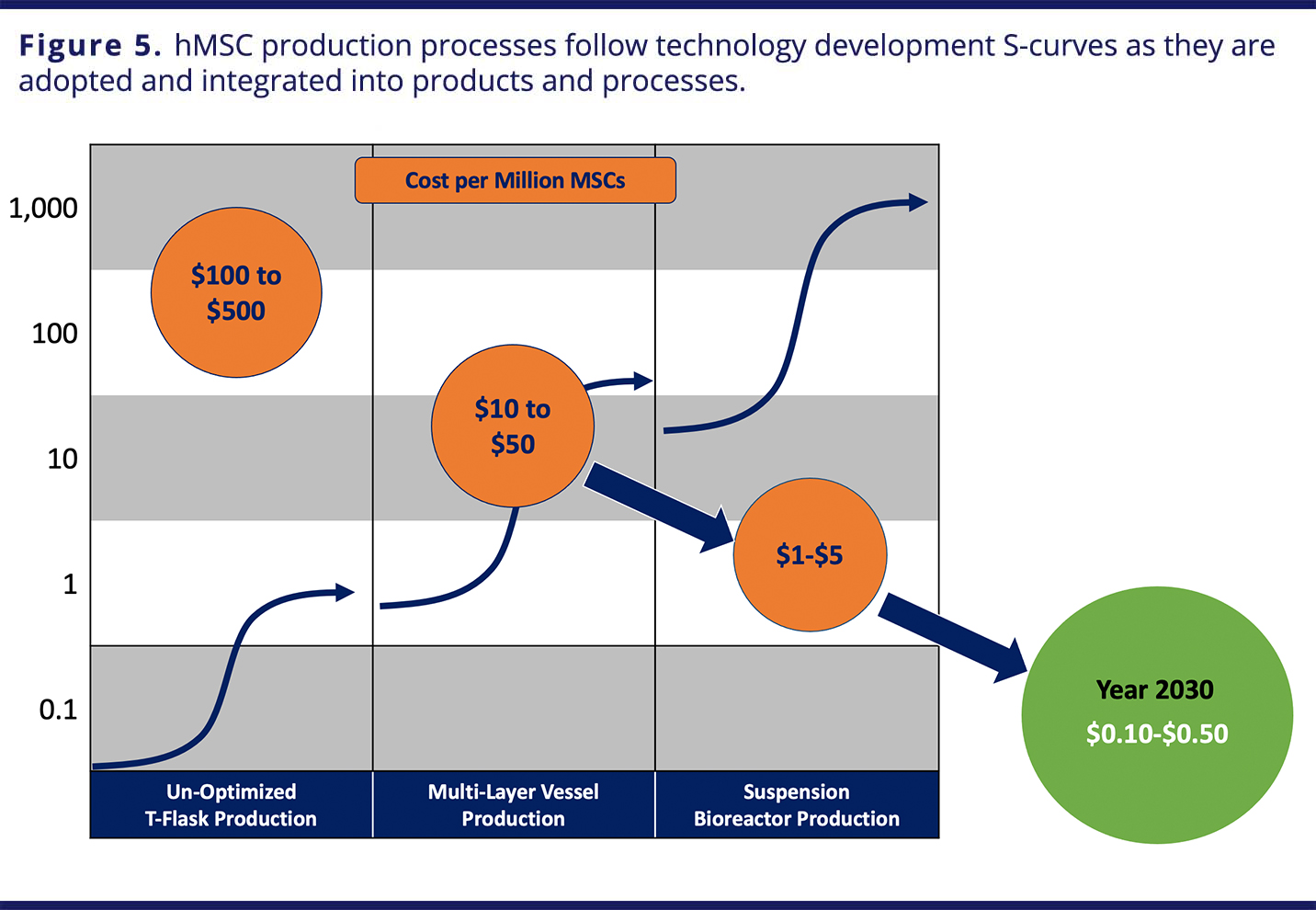
As manufacturing breakthroughs enable larger-scale production to increase the availability of hMSCs and drive down their cost due to efficiencies of scale, further innovation will follow (Figure 5).
References
- Lembong, Josephine, Jon Carson and Jon Rowley. “The Stabilization of hMSCs as a Technology.” RoosterBio® White Paper. 2020.
- Olsen, Timothy R., Kelvin S. Ng, Lye T. Lock, Tabassum Ahsan and Jon A. Rowley. “Peak MSC—Are We There Yet?” Frontiers in Medicine. 5:178 (2018).
- Maris B. "Medicine’s transistor moment: 8 emerging technologies that could revolutionize the life sciences." Medium (2015) Available online at: https://www.speakingtree.in/article/medicine-s-transistor-moment
- The Drug Development Process. (2015) Available online at: http://www.fda.gov/forpatients/approvals/drugs/default.htm
- Nguyen, BNB, Ko H, Moriarty RA, Etheridge JM, Fisher JP. “Dynamic bioreactor culture of high volume engineered bone tissue.” Tissue Eng. Part A. 22:263–71 (2016).
- Ziegler-Graham, K, MacKenzie EJ, Ephraim PL, Travison TG, & Brookmeyer R. “Estimating the Prevalence of Limb Loss in the United States: 2005 to 2050.” Phys. Med. Rehabil. 89:422–9 (2017).
- Organ Donation Statistics. U.S. Department of Health and Human Services. 2017. Available online at: https://www.organdonor.gov/statistics-stories/html
- Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, et al. “Recent advances in 2D and 3D in vitrosystems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME.” Arch Toxicol. 87:1315–530 (2013).
- Chan C, Berthiaume F, Nath BD, Tilles AW, Toner M, Yarmush ML. “Hepatic tissue engineering for adjunct and temporary liver support: critical technologies.” Liver Transplant. 10:1331–42 (2004).
- Wang B, Zhao L, Fish M, Logan CY, & Nusse R. “Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver.” Nature. 524:180–5 (2015).
- Clavien PA, Petrowsky H, DeOliveira ML, & Graf R. “Strategies for safer liver surgery and partial liver transplantation.” Engl. J. Med. 356:1545–59 (2007).
- Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F, et al. “An estimation of the number of cells in the human body. Hum. Biol. 40:463–71 (2013).
- Chan C, Berthiaume F, Nath BD, Tilles AW, Toner M, & Yarmush ML. Hepatic tissue engineering for adjunct and temporary liver support: critical technologies. Liver Transplant. 10:1331–42 (2004).
- Von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, et al. “Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation.” Stem Cells. 30:1575–8 (2012).
- Gnecchi M, Zhang Z, Ni A, & Dzau VJ. “Paracrine mechanisms in adult stem cell signaling and therapy.” Res. 103:1204–19 (2008).
- Ng KS, Kuncewicz TM, & Karp JM. “Beyond Hit-and-Run: Stem Cells Leave a Lasting Memory.” Cell Metab. 22:541–3 (2015). doi:
- Rani S, Ryan AE, Griffin MD, & Ritter T. “Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications.” Mol Ther. 23:812–23 (2015).
- Liang X, Ding Y, Zhang Y, Tse HF, & Lian Q. “Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives.” Cell Transplant. 23:1–32 (2013).
- Parekkadan B, Van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, et al. “Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure.” PLoS ONE. 2:e941 (2007).
- Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, et al. “Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury.” Sex. Med. 7:3331–40 (2010).
- Wood L. Global Cosmeceuticals Market Worth USD 61 Billion by 2020 - Analysis, Technologies & Forecasts Report 2016-2020 - Key Vendors: Avon, Bayer, Johnson & Johnson - Research and Markets. 2016.
- van der Weele C & Tramper J. “Cultured meat: Every village its own factory?” Trends Biotechnol. 32:294–6 (2014).
- Olsen TR, Lock LT, & Rowley JA. “Scaling up: how manufacturing sciences will dictate the future of cell therapy.” Med.11:S15–8 (2016).
- Rowley, J.A. and S.A. Montgomery “The Need for Adherent Cell Manufacturing: Production Platform and Media Strategies Drive Cell Production Economics.” BioProcess International 16:34-49 (2018).
*This article draws heavily (including the republished figures) from the white paper “Peak MSC — Are We There Yet?” by Timothy R. Olsen, Kelvin S. Ng, Lye T. Lock, Tabassum Ahsan, and Jon A. Rowley published in 2018 in Frontiers in Medicine.
