June 18, 2021 PAO-06-21-CL-07
The project described here involved creating a new large production line for BFS containers with a filling volume of 100 mL. As VITRONIC has the expertise required, the company was tasked with designing and implementing an inspection solution for the new production line.
A central aspect of the solution implemented is that the inspection is “static.” The product passes through the machine without needing to be turned, tilted, or presented in any other form that is not essential from a manufacturing perspective. This is possible thanks to the use of 13 individual cameras, including a special 360° lens that was customized for the product. Both black-and-white and color cameras are used in the machine. This setup implements 13 different inspection modules, which inspect the individual defect classes.
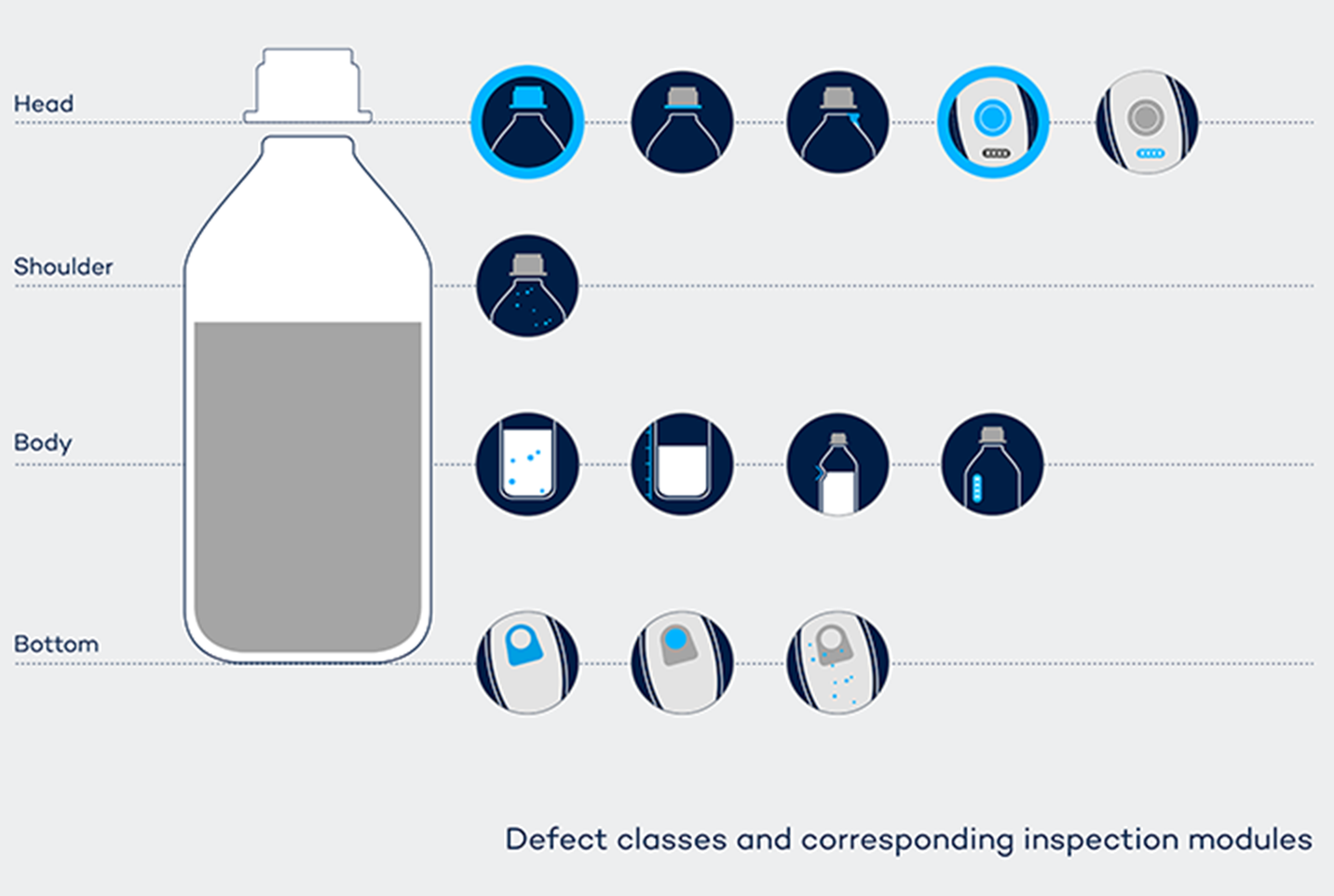
In terms of software, each of the inspection modules has its own evaluation algorithm, capable of checking the image material for the correct properties.
Accordingly, 13 individual images are captured of each BFS container that is manufactured. With a resolution of up to approximately 2500 × 2000 pixels per camera, a large volume of data is created for each container.
In the filling area, it is critical that the inspection solution is fully integrated into the filling system, with a single machine control interface. As a result, any containers identified as defective can be filtered out–even before they leave the filling area, so that they do not reach the downstream sterilization and packaging areas. Finally, all defect classes are inspected in the packaging area.

With the automated visual inspection solution from VITRONIC, the new production line in the packaging area achieves a maximum output of 14,400 containers per hour in accordance with the customer specifications. This represents an increase of 60% compared with the previous line. “The greatest benefit for our client is that we can conduct a 100% visual inspection without losing any time and with a performance rate that was not not previously achieved at any other site,” says Frank Fohler, Deputy Head of Healthcare Department at VITRONIC. “This enabled the client to save the cost of one entire machine at the site.”
The system used here is based on results databases, which are fed with data by the evaluation algorithms that can easily be exported. Engineers in charge of production can examine these defect image memories, the analysis data, and the corresponding reject rates in order to optimize the relevant process step in the system.
The second benefit of the new system for the client is due to the advanced degree of system harmonization it enables with a further solution from VITRONIC added to the site. The workers at the plant are already very familiar with VITRONIC and are glad to be using a unified system. The company can, in turn, boost its efficiency in terms of human resources. Staff with expertise in one type of inspection system can, if necessary, apply this knowledge to all VITRONIC systems in use.
The decisive, market-relevant benefit of the solution results from integrating inspection into the filling system in a way that is synchronized with the production cycle.
This aspect of the setup alone has the potential to significantly boost production efficiency – without it, all defective parts could only be removed at the packaging stage, involving a proportionately greater degree of effort (“scrap refinement”).
“Other conceivable solutions for the filling area, such as using a separate machine for the inspection, are costly by comparison. In this case, additional buffer segments would be required, in turn making the production processes significantly longer,” says Frank Fohler.
To sum up, Frank Fohler says, “The new inspection system at the production plant is a fully integrated solution and saves valuable cycle time. This is the cleanest possible solution.”

Nice Insight, established in 2010, is the research division of That’s Nice, A Science Agency, providing data and analysis from proprietary annual surveys, custom primary qualitative and quantitative research as well as extensive secondary research. Current annual surveys include The Nice Insight Contract Development & Manufacturing (CDMO/CMO), Survey The Nice Insight Contract Research - Preclinical and Clinical (CRO) Survey, The Nice Insight Pharmaceutical Equipment Survey, and The Nice Insight Pharmaceutical Excipients Survey.

VITRONIC is a global leader in the field of industrial machine vision headquartered in Wiesbaden, Germany. VITRONIC supports customers in over 60 countries via a global network of subsidiaries, service centers, and partner companies. The group is covering a wide spectrum from standard products with customer-specific upgradable modules to individually customized solutions in its core sectors of the healthcare and automotive industries, logistics, and traffic technology.
VINSPEC HEALTHCARE offers an integrated solution for automated visual inspection of pharmaceutical packaging. The quality inspection system covers all requirements throughout the entire packaging process. These include inline inspection of empty containers, as well as inspections during the filling and sealing processes. The portfolio includes fully automated inspection solutions for vials, BFS, IV bags, TTS, and insulin pens. Systems can be flexibly integrated into existing production lines.