October 26, 2018 PAP-Q4-18-NI-004
The Innovation Issue Feature: Part 3
Integrating data into all phases of the life cycle means there will be a measurable push in distribution and packaging, as much as in development and manufacturing. Considering the FDA mandate for serialization, the agency is basically setting up the pharma distribution supply chain for the next wave in big data. At its core, the mandate will make track-and-trace solutions, and therefore data visualization, a must for the industry going forward.
The move toward product tracking by the FDA involves a number of elements, including information about all parties that have handled a drug each time it is sold in the U.S. market, as well as a wealth of tracing information that is easily available and transparent.1 This tracing information is defined as the drug product’s transaction information (TI), transaction history (TH) and transaction statement (TS).1
The TI includes all details about the drug, including name, strength, lot number, national drug code, container size, number of containers, date of the transaction, date of the shipment (if more than 24 hours after the date of transaction) and the business name and address of the person to whom ownership is being transferred.1 The TH is a detailed account of all transaction information that begins with the drug manufacturer and involves all subsequent parties.1 The TS is a written statement that assures that the entity assuming ownership in the transaction is authorized under the DSCSA (Drug Supply Chain Security Act) and has received all transaction information before receipt, has not altered any history, is in compliance with all verification systems and did not knowingly ship a suspect product.1
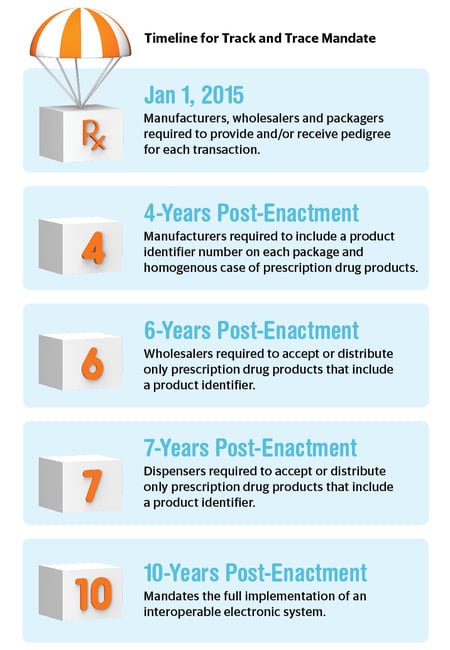
This translates to a huge amount of data that must be available and accounted for at all levels, as part of the FDA plan to make sure all drugs are trackable and traceable in accordance with the agency’s ongoing serialization effort. After the serialization mandate officially goes into effect, only products with encoded product identifiers are to be bought and sold. This will take place on November 2018 for repackers, November 2019 for wholesale distributors and November 2020 for dispensers. By 2023, the FDA is pushing for enhanced product tracing at the package level.1
To prepare for the upcoming emphasis on track and trace, manufacturers have altered their internal operations to make sure that they are compliant. In doing so, all drug products must be serialized to meet the specifications of the FDA mandate. To comply with these outlined requirements, contract development and manufacturing organizations (CDMOs) must add a unique 2D barcode (the GS1 DataMatrix) to their drug containers.2 These barcodes convey the significant attributes of the drug product and thus integrate data into the supply chain.2 This is mainly being done to stop counterfeiting and allow only legitimate drug products on the market, as a way to increase public safety and health overall.1
For track and trace to be correctly implemented, a software solution is needed. A track-and-trace software solution must allow data to be received and shared easily. It must also be used as an internal security check, confirming that all serial numbers are original and are accounted for within the system.3 An ideal system will go beyond the mandated requirements and manage the serialization data being recorded. These data can then be applied at the machine level and on to a management level, before ultimately being shared and merged into a larger governmental database network.
Taking this into consideration, it is obvious that the industry-wide serialization effort fits well within the trend of data integration. Serialization has forced companies to modify internal operations to be more data compliant, such that an outside organization can also read and recognize their data, which can then be gathered as a part of a larger framework. By mandating barcodes, the FDA has made sure that the industry is considering data, going so far as to visualize and implement it, so that packaging operations are reliant on data for distribution to move forward.
As Justin Schroeder, Senior Executive Director, Global Marketing & Design, PCI Pharma Services, wrote in an article on “Ensuring Serialization Effectiveness” for Contract Pharma Magazine, serialization has put pressure on the industry to reform.4 “With the advent of serialization, and each package being unique, this systematic approach inherently requires electronic data capture and submission to downstream partners,” wrote Schroeder. “Traditional coding techniques such as embossing/debossing, or older inkjet technologies, do not provide a verifiable presence and resolution to be effectively verified through modern vision inspection for code verification and subsequent commissioning and aggregation activities vital to Serialization implementation.”4
The stress on serialization and thus on software for coding solutions is a tangible move toward making post-market data mining more robust. This information will be cycled back into new drug development and may also used as a measure of adherence down to the patient level. Though the FDA has mandated a barcode on packaging, some companies are taking track and trace a step further by placing a serialized code on each pill manufactured.
Serializing drugs down to the pill could be an effective step in the national crisis of nonadherence. Nonadherence to medicine poses a risk to patient safety and contributes to a burden in the industry. It is estimated that medication nonadherence costs the healthcare system between $100 and $289 billion a year.5 Track and trace will play a part in assuaging this issue, if not from a visual redesign that makes it easier for patients to understand their drugs, then through more aggressive means, such as an ingestible sensor that is able to confirm if a patient has taken their medicine. This is an option that companies have already begun testing.6
Apps and mobile solutions are also being linked to medicines at the patient level. Again, this is adding to a big data arsenal of real-time information regarding not only adherence, but also tolerability and side effects. The industry has reached a point where patients can be accessed and communicated with at all times. This will no doubt impact how trials are conducted and how drugs are made, but also how drugs are monitored and how patient follow-up is performed once a drug is actually on the market. This will also provide a direct channel for doctors and providers to access patients and monitor their treatment in greater depth.
In a study titled “Using Artificial Intelligence to Reduce the Risk of Nonadherence in Patients on Anticoagulation Therapy” published in May 2017, an AI smart phone program was directly correlated to increased adherence, and even patients with less experience using a smart phone were still able to master this technology.7 According to the study, “50% demonstrated a improvement in adherence based on plasma drug concentration levels,” while, for those who were receiving direct oral anticoagulants, the “absolute improvement” rose to 67%. The study results concluded that, “real-time monitoring has the potential to increase adherence and change behavior, particularly in patients on direct oral anticoagulant therapy.”7 The artificial intelligence platform was easily accessible on patient mobile devices through an app. The app visually identified the patient and the medication through an algorithm and confirmed medication ingestion.
The app provided patients with reminders and instructions for dosing. If a dose was taken late, a notification was triggered within the hour and before the end of the dosing window. The app sent real-time data to web-based dashboards where staff received an automated message if a dose was missed, late, or not properly taken.7 The AI app data conformed to five categories: 1) visual confirmation of ingestion, 2) self-reported dose via the AI app, 3) self-reported dose by clinic staff, 4) missed dose and 5) dose taken in the clinic.7
The study concluded: “Absolute improvement of 67% in patients taking DOACs and monitored by the AI app — and confirmed by drug levels — demonstrates the potential value of daily real-time monitoring to measure and maximize adherence…. Artificial Intelligence platforms have the potential to accurately monitor medication ingestion and change patient behavior.”7
This experiment proves that there is no area of the industry that data integration and AI cannot transform. The implementation of data across the life cycle will change the way drugs are developed, manufactured and even adhered to. Data has the ability to impact the entire supply chain from start to finish. In order for a pharmaceutical, biotech or facility engineering company to succeed in the current and immediate landscape, an emphasis on the future of drug development and a heightened focus on harnessing the power of data throughout will be requirements. This will also signify a change in mindset and a shift in thinking from surface-level production and output to theorizing around data that is driving production. Data points will be measured and utilized as part of a bigger-picture strategy that offers benefits for all share- and stakeholders involved.

Emilie is responsible for strategic content development based on scientific areas of specialty for Nice Insight research articles and for assisting client content development across a range of industry channels. Prior to joining Nice Insight, Emilie worked at a strategy-based consulting firm focused on consumer ethnographic research. She also has experience as a contributing editor, and has worked as a freelance writer for a host of news and trends-related publications