The growth of decentralized, virtual, and hybrid clinical trials was accelerated immensely by the COVID-19 pandemic, as pharmaceutical companies around the world were forced to turn to alternative solutions to continue their research in the face of unprecedented mobility challenges. As restrictions continue to ease,
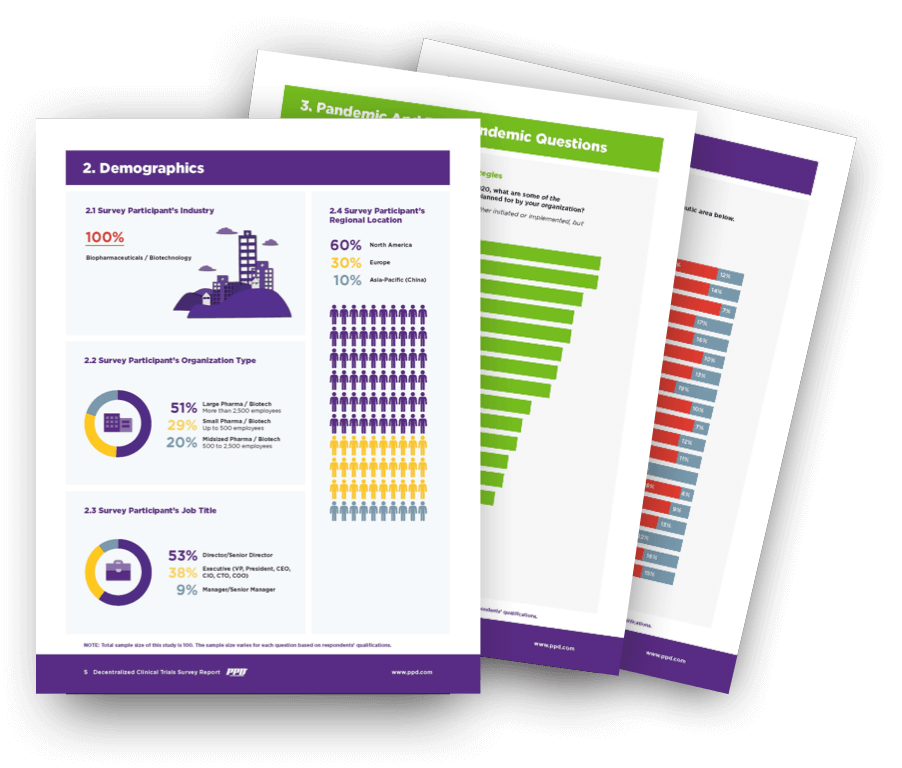
Pharma’s Almanac, Nice Insight, and PPD (part of Thermo Fisher Scientific) collaborated to explore what a post-pandemic future holds for the continued growth of decentralized and hybrid clinical trials.
A global cross-section of pharmaceutical leaders weighed in on:
- How decentralized and hybrid trial solutions met their expectations
- Future plans for decentralized and hybrid clinical trials
- The comparison between data quality from decentralized trials and traditional in-person trials
- The most desired benefits from decentralized and hybrid trials
- Interest in being an early adopter of new techniques, products, and platforms for decentralized trials
A special thanks to PPD for sponsoring this effort and making the information available to the entire Pharma’s Almanac Community.