October 26, 2018 PAP-Q4-18-NI-005
Integrating data from the drug development phase through drug manufacturing presents an additional set of challenges. As with drug development, the infrastructure needed to manage the data is a major hurdle. The question of how to handle big data — and what to do with it — is a key concern when dealing with such a high level of information on all fronts and throughout all phases of the life cycle. Specifically, if big data is integrated into manufacturing, it must be thoroughly managed such that it contributes seamlessly to daily operations.

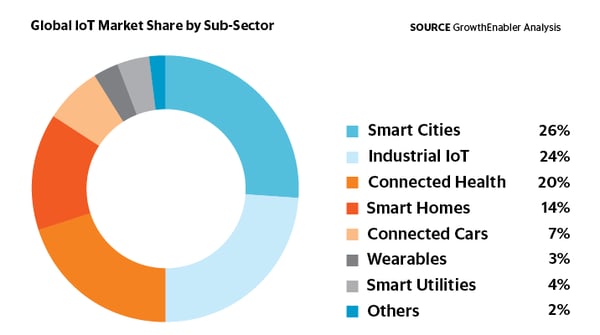
Data integration on the factory floor will be achieved by relying on the Internet of Things (IoT), which is defined as the interconnectedness of everyday computing devices, enabled with the Internet to send and receive data.1 This network of smart objects is growing by the minute. Analyst firm Gartner estimates that by 2020 there will be more than 26 billion connected devices, although other estimates put this number closer to 100 billion.1 The IoT is a connected network that includes people and their relationships with their tech and each other. Relationships fit into the following framework: device to device, device to person and person to person.
The IoT paints a picture of a not so distant tomorrow in which smart devices are embedded in every aspect of our world, communicating constantly and exchanging information in a measurable way. Perhaps the best example of the wide-reaching impact of the IoT put into practice is Google’s “city of the future” or “smart city.” The company is building a city that will be entirely structured around device-to-person and IoT-enabled interconnected relationships. Machine learning and machine connection will make it possible for this experimental city to update and upgrade itself in real time based on user feedback and analysis of patterns and behavior, picked up through tracking and data analysis. Google’s smart city, known as Quayside, is currently in development to be built in Toronto.2
Considering an entire city can be built on data and algorithms, applying this model to the manufacturing floor seems like far less of a stretch, while also offering the same opportunity for efficiency and effectiveness. Data built into operations can ideally self-correct and re-route or transform processes in ways that may have previously gone unnoticed. The ability to track commercial operations as they occur in real time presents an unprecedented opportunity for cost savings and increased efficiency on the manufacturing floor.
Smart manufacturing put into practice holds tremendous potential. Data are already heavily embedded in process development and optimization, though a plant remodeled to run on algorithms with an emphasis on the IoT would take data integration to the next level. Quality control teams must analyze and collect data at every step of the manufacturing process —from when the drug is still considered raw material to its packaging and distribution — ensuring that it is fit for human consumption and has been processed correctly throughout the life cycle. This results in a tremendous amount of information, but it is not necessarily embedded in the organization, as it would be in a smart facility.
In order to improve the accuracy and implementation of data throughout the life cycle, smart devices must be employed. These devices will extend to fully automated systems. According to Jack Schmidt, SAP Life Science Industry Director, who spoke on the trend of big data in an article for Pharmaceutical Online, data integration into smart facilities has the ability to transform the entire industry.1 “With the power of in-memory computing technology and interconnected and automated systems, manufacturers now have the ability to analyze these massive amounts of quality, environmental, and IoT-generated factory data,” wrote Schmidt. “Tapping into this Big Data allows companies to build end-to-end process controls, resulting in higher-quality products, more predictability, more efficient manufacturing, and faster time to market.”1
In order to put this big data into practice, however, an integrated platform must be built into operations. Data with no grounding or context is essentially meaningless — if it is not being used or translated in a real way, then it is just a mass of useless information. Dubbed “islands of data,” information that lives on its own without any link to the overarching process cannot bring an organization the maximum amount of integrated value through data generation. Even if it is useful in practice with individual phases in manufacturing or development, there are bigger-picture issues that are missing, with broader questions yet to be addressed. This includes questions about gaps in manufacturing, or lack of robustness, the cost of downtime and the demand for different materials, as well as the production winner in a network of facilities.3 In essence, a central unifying platform that is able to analyze, translate and predict data going forward is crucial to remedying gaps in operations, connecting data islands and increasing yields in production.
In an article in The Medicine Maker on “Embracing the Digital Enterprise,” Bob Dvorak, Ph.D., Principal at BioPharma Data & Manufacturing Systems Consulting, and Rick Johnston, Ph.D., Founder and CEO of Bioproduction Group, explained this platform as a digital enterprise, including what is needed for its success.3 “The digital enterprise builds on the automation systems you already have in place and allows users to see how a change in one area of the facility cascades through other processes and areas via a complex set of relationships and business rules. Identifying the pattern of cause and effect up front enables the business to see the impact of a change or adverse event not just on a single manufacturing area, but on overall metrics like adherence to plan and cost per batch,” they noted.3
“The foundation of the digital enterprise starts with capturing your process knowledge to create an overall ‘map’ of the process that provides context for data. The map gets populated with data from your manufacturing runs, with knowledge from your experts and, coupled with your historical data, provides a foundation of certainty about what you will be seeing. This approach allows you to tunnel into the unit operations in a meaningful way: not mining data, but populating process models with actual numbers,” continued the explanation.3
Taking the data into context and using these numbers to better inform strategy and thus turning it into actionable results will lead to an overall improved process. For this to be a truly integrated system, all process must be contained in the same digital platform. This platform must operate in real time to be effective, with decisions made on the basis of the data generated. All bases of data must be covered, with data being combined to register what is or is not working and how, in order to bolster a bottom line and increase profitability.3
Gathering this data is not necessarily going to be easy, but the benefits are clear. It must be a group effort, with the entire life cycle uniting for the goal of improving process via data generation. Ultimately, automated IoT sensors and a complete algorithm that generates a cohesive picture of the data and process inefficiencies (and efficiencies) will be key to supporting this type of next-generation facility.
Big data has already begun to affect pharmaceutical manufacturing, with companies beginning to capitalize by employing these logistics in operations. An often-cited case study involves a European specialty chemicals manufacturer that employed neural-network techniques (analytics that mimic brain activity) in order to compare production inputs and yields.4 Certain sensitivities that had previous slipped by the company’s notice were pointed out. The company adjusted its parameters according to these results and increased production by reducing waste and energy costs.4
The results made possible by integrating data into production demand investment. This is exactly what is happening as the industry becomes poised for data integration on all levels: as a tool in drug development and a pivotal stepping stone to precision medicine, as well as a way to reduce the cost of drug development and improve production and overall outcomes in manufacturing. According to a report by the firm SNS Research, big data investment is on track to grow at a compound annual growth rate of 15% over the next three years and will account for more than $5.8 billion in spending by the end of 2020.5 The report also measured cost savings related to data integration, with hospitals and other healthcare facilities achieving significant cost reductions of “more than 10%, as well improvements in outcomes by as much as 20% for certain conditions, growth in revenue by 30%, and increase in patient access to services by more than 35%,” as a result of the implementation of actionable data across operations.5
The company that succeeds in successful data integration will no doubt benefit across the life cycle; the challenge, however, is getting there. To achieve cohesive data integration in drug manufacturing, systems must be aligned. The IoT must be implemented, with devices communicating with one another in order to eliminate gaps in production. The “islands of data” must be closed by one system — an integrated platform that can unite production such that it runs as a cohesive digital enterprise. This platform must be programmed to constantly upgrade and update itself, with company decision makers relying on real-time data to create actionable results and improve process yields and manufacturing conditions, eliminate cost and waste and generate a higher rate of returns, to make the smart facility of the future a reality of today.

David is Scientific Editor in Chief of the Pharma’s Almanac content enterprise, responsible for directing and generating industry, scientific and research-based content, including client-owned strategic content, in addition to serving as Scientific Research Director for That's Nice. Before joining That’s Nice, David served as a scientific editor for the multidisciplinary scientific journal Annals of the New York Academy of Sciences. He received a B.A. in Biology from New York University in 1999 and a Ph.D. in Genetics and Development from Columbia University in 2008.