That's Nice recommends a list of databases that will aid CDMOs and CROs in becoming industry leaders.
Quality has evolved from focusing on the what (defining the product) to the how (improving how it is produced) over the past years.
The first step in establishing an effective process safety strategy is determining the company’s capabilities & limitations—with respect to process hazards.
Most research indicates that only 40% to 60% of mergers succeed — and just 30% are cross-border mergers (Association for Corporate Growth).
An effective capital equipment investment recovery strategy can turn idled equipment into money-making assets, and network redeployments that save money.
A commitment to transparency, open communication, and a genuine respect for the needs of each client’s individual projects are essential for CDMOs to succeed.
Biopharmaceutical CDMOs must have extensive knowledge about the impact of process conditions on product characteristics.
Results observed in clinical trials do not always translate into what is being experienced by consumers in the marketplace, key reason - non-adherence.
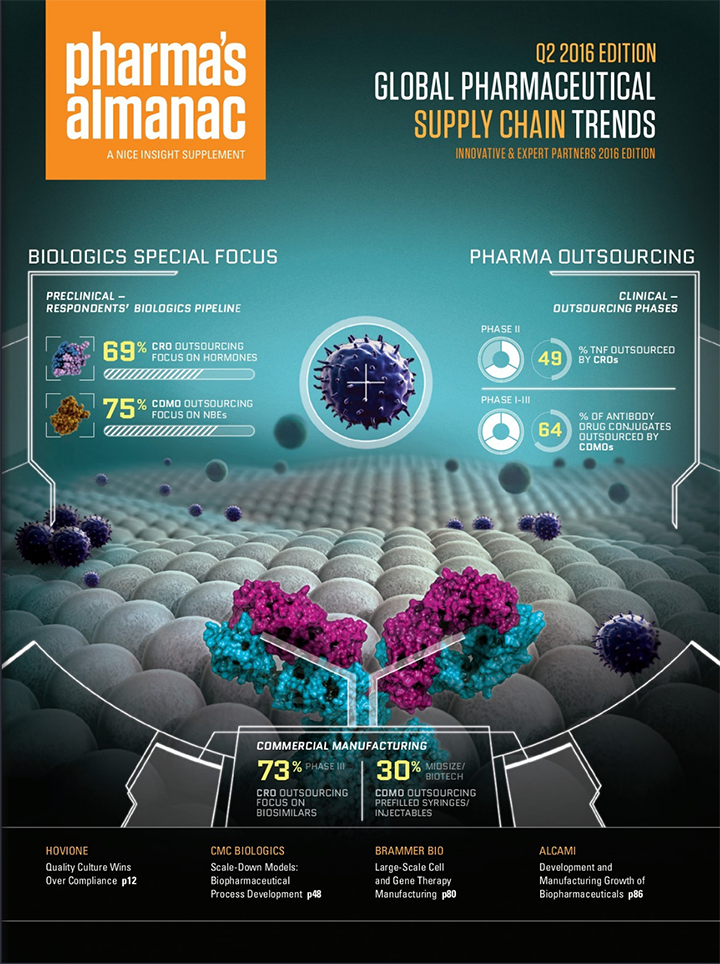
According to the 2016 Nice Insight CDMO Outsourcing Survey, about two-thirds of companies are developing large-molecule products as new biological entities (NBEs).
Innovators with new molecules are looking for their supply chain partners to deliver innovative technologies to make chemicals more soluble.
ADCs are a huge challenge in terms of manufacturing and capital investment because a company needs to build both biologic and chemical synthesis capabilities.
Demand for injectable drug products is increasing, due to growth of the biologics market & injectable formulations offering a mechanism for increased efficacy.
The 2016 Nice Insight Pharmaceutical Equipment Survey was deployed to around 489 industry professionals worldwide.
According to the 2016 Nice Insight CDMO Survey, nearly 70% of outsourced projects were sent to a combination of preferred providers & strategic partners.
Earlier held misconceptions about biocatalysis such as yield, reliability, scalability are now readily addressable by enzyme engineering & process development.
"Connected at Every Level" communicates integration through science and philosophy.
With options for both upstream and downstream processes, the flexible factory concept is becoming the model for cost-effective, aseptic production.
Scale-down models are increasingly adopted by the industry & serve as an indispensable tool for process development, characterisation & validation.
To attract and keep customers, competitive CDMOs are bolstering their offerings by expanding areas of expertise through forming strategic partnerships.
To speed up drug development, organisations are integrating discovery & development by conducting interdisciplinary studies in formulation development & DMPK.
The global biopharmaceuticals market valued at $162 billion in 2014, is predicted to grow at a compound annual growth rate of 9.4% from 2014 to 2020.
Much of biosimilars’ market potential will depend on insurers' willingness to pay for these medications & pricing will be similar to current biologics pricing.
A rich pipeline of over 500 cell and gene therapy products currently in the clinical trials, will drive significant capacity needs in the future.
Brammer's brand identity was created with a visual allusion to cells.